Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system
- PMID: 15071119
- PMCID: PMC6729751
- DOI: 10.1523/JNEUROSCI.0188-04.2004
Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system
Abstract
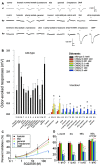
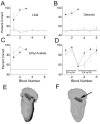
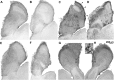
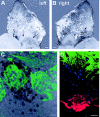
It is believed that odor transduction in the mammalian main olfactory system only involves the cAMP-signaling pathway. Here, we report on odor responsiveness in mice with a disrupted cyclic nucleotide-gated (CNG) channel subunit A2. Several odorants, including putative pheromones, can be detected and discriminated by these mice behaviorally. These odors elicit responses in the olfactory epithelium, main olfactory bulb, and olfactory (piriform) cortex of CNGA2 knock-out mice. In addition, responses to odors detected by CNGA2 knock-out mice are relatively insensitive to inhibitors of the cAMP pathway. These results provide strong evidence that cAMP-independent pathways in the main olfactory system of mammals participate in detecting a subset of odors.
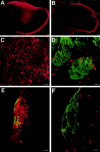
Figures


Similar articles
-
Deficits in sexual and aggressive behaviors in Cnga2 mutant mice.Nat Neurosci. 2005 Dec;8(12):1660-2. doi: 10.1038/nn1589. Epub 2005 Oct 30. Nat Neurosci. 2005. PMID: 16261133
-
Importance of the CNGA4 channel gene for odor discrimination and adaptation in behaving mice.Proc Natl Acad Sci U S A. 2003 Apr 1;100(7):4299-304. doi: 10.1073/pnas.0736071100. Epub 2003 Mar 20. Proc Natl Acad Sci U S A. 2003. PMID: 12649326 Free PMC article.
-
Visualization of odor-induced neuronal activity by immediate early gene expression.BMC Neurosci. 2012 Nov 5;13:140. doi: 10.1186/1471-2202-13-140. BMC Neurosci. 2012. PMID: 23126335 Free PMC article.
-
Emerging views on the distinct but related roles of the main and accessory olfactory systems in responsiveness to chemosensory signals in mice.Horm Behav. 2004 Sep;46(3):247-56. doi: 10.1016/j.yhbeh.2004.02.009. Horm Behav. 2004. PMID: 15325226 Review.
-
Mammalian pheromones.Annu Rev Physiol. 2014;76:151-75. doi: 10.1146/annurev-physiol-021113-170334. Epub 2013 Aug 26. Annu Rev Physiol. 2014. PMID: 23988175 Free PMC article. Review.
Cited by
-
Olfactory and solitary chemosensory cells: two different chemosensory systems in the nasal cavity of the American alligator, Alligator mississippiensis.BMC Neurosci. 2007 Aug 3;8:64. doi: 10.1186/1471-2202-8-64. BMC Neurosci. 2007. PMID: 17683564 Free PMC article.
-
Olfaction and Pheromones: Uncanonical Sensory Influences and Bulbar Interactions.Front Neuroanat. 2017 Nov 15;11:108. doi: 10.3389/fnana.2017.00108. eCollection 2017. Front Neuroanat. 2017. PMID: 29187814 Free PMC article. Review.
-
The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice.Eur J Neurosci. 2006 Jan;23(2):521-30. doi: 10.1111/j.1460-9568.2005.04589.x. Eur J Neurosci. 2006. PMID: 16420459 Free PMC article.
-
Accessory olfactory bulb function is modulated by input from the main olfactory epithelium.Eur J Neurosci. 2010 Mar;31(6):1108-16. doi: 10.1111/j.1460-9568.2010.07141.x. Epub 2010 Mar 8. Eur J Neurosci. 2010. PMID: 20377623 Free PMC article.
-
Genetic dissection of pheromone processing reveals main olfactory system-mediated social behaviors in mice.Proc Natl Acad Sci U S A. 2015 Jan 20;112(3):E311-20. doi: 10.1073/pnas.1416723112. Epub 2015 Jan 6. Proc Natl Acad Sci U S A. 2015. PMID: 25564662 Free PMC article.
References
-
- Ache BW, Zhainazarov AB (1995) Dual second-messenger pathways in olfactory transduction. Curr Opin Neurobiol 5: 461–466. - PubMed
-
- Andreolini F, Jemiolo B, Novotny M (1987) Dynamics of excretion of urinary chemosignals in the house mouse (Mus musculus) during the natural estrous cycle. Experientia 43: 998–1002. - PubMed
-
- Baker H, Morel K, Stone DM, Maruniak JA (1993) Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res 614: 109–116. - PubMed
-
- Belluscio L, Gold GH, Nemes A, Axel R (1998) Mice deficient for Golf are anosmic. Neuron 20: 69–81. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
