Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport
- PMID: 15071184
- PMCID: PMC395893
- DOI: 10.1073/pnas.0308456101
Genetic evidence for nonredundant functional cooperativity between NPC1 and NPC2 in lipid transport
Abstract
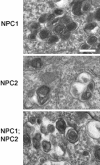
Niemann-Pick C (NPC) disease is a fatal neurodegenerative disorder characterized by a lysosomal accumulation of cholesterol and other lipids within the cells of patients. Clinically identical forms of NPC disease are caused by defects in either of two different proteins: NPC1, a lysosomal-endosomal transmembrane protein and NPC2, a soluble lysosomal protein with cholesterol binding properties. Although it is clear that NPC1 and NPC2 are required for the egress of lipids from the lysosome, the precise roles of these proteins in this process is unknown. To gain insight into the normal function of NPC2 and to investigate its interactions, if any, with NPC1, we have generated a murine NPC2 hypomorph that expresses 0-4% residual protein in different tissues and have examined its phenotype in the presence and absence of NPC1. The phenotypes of NPC1 and NPC2 single mutants and an NPC1;NPC2 double mutant are similar or identical in terms of disease onset and progression, pathology, neuronal storage, and biochemistry of lipid accumulation. These findings provide genetic evidence that the NPC1 and NPC2 proteins function in concert to facilitate the intracellular transport of lipids from the lysosome to other cellular sites.
Figures
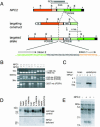
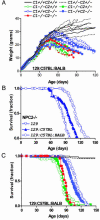
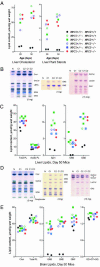
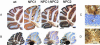

References
-
- Ioannou, Y. A. (2001) Nat. Rev. Mol. Cell Biol. 2, 657–668. - PubMed
-
- Davies, J. P., Chen, F. W. & Ioannou, Y. A. (2000) Science 290, 2295–2298. - PubMed
-
- Okamura, N., Kiuchi, S., Tamba, M., Kashima, T., Hiramoto, S., Baba, T., Dacheux, F., Dacheux, J. L., Sugita, Y. & Jin, Y. Z. (1999) Biochim. Biophys. Acta 1438, 377–387. - PubMed
-
- Zervas, M., Somers, K. L., Thrall, M. A. & Walkley, S. U. (2001) Curr. Biol. 11, 1283–1287. 86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

