Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography
- PMID: 15071499
- PMCID: PMC394246
- DOI: 10.1038/sj.emboj.7600192
Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography
Abstract
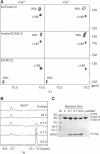
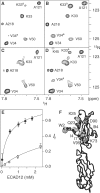
Cellular adhesion by classical cadherins depends critically on the exact proteolytic removal of their N-terminal prosequences. In this combined solution NMR and X-ray crystallographic study, the consequences of propeptide cleavage of an epithelial cadherin construct (domains 1 and 2) were followed at atomic level. At low protein concentration, the N-terminal processing induces docking of the tryptophan-2 side-chain into a binding pocket on the same molecule. At high concentration, cleavage induces dimerization (KD=0.72 mM, k(off)=0.7 s(-1)) and concomitant intermolecular exchange of the betaA-strands and the tryptophan-2 side-chains. Thus, the cleavage represents the switch from a nonadhesive to the functional form of cadherin.
Figures


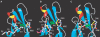

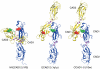
References
-
- Alattia JR, Ames JB, Porumb T, Tong KI, Heng YM, Ottensmeyer P, Kay CM, Ikura M (1997) Lateral self-assembly of E-cadherin directed by cooperative calcium binding. FEBS Lett 417: 405–408 - PubMed
-
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L (2002) C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296: 1308–1313 - PubMed
-
- CCP4 (1994) Collaborative Computing Project No. 4. The CCP4 suite: programs for protein crystallography. Acta Crystallogr D 50: 760–763 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

