Trypanothione S-transferase activity in a trypanosomatid ribosomal elongation factor 1B
- PMID: 15073172
- PMCID: PMC3428924
- DOI: 10.1074/jbc.M311039200
Trypanothione S-transferase activity in a trypanosomatid ribosomal elongation factor 1B
Abstract
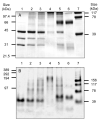
Trypanothione is a thiol unique to the Kinetoplastida and has been shown to be a vital component of their antioxidant defenses. However, little is known as to the role of trypanothione in xenobiotic metabolism. A trypanothione S-transferase activity was detected in extracts of Leishmania major, L. infantum, L. tarentolae, Trypanosoma brucei, and Crithidia fasciculata, but not Trypanosoma cruzi. No glutathione S-transferase activity was detected in any of these parasites. Trypanothione S-transferase was purified from C. fasciculata and shown to be a hexadecameric complex of three subunits with a relative molecular weight of 650,000. This enzyme complex was specific for the thiols trypanothione and glutathionylspermidine and only used 1-chloro-2,4-dinitrobenzene from a range of glutathione S-transferase substrates. Peptide sequencing revealed that the three components were the alpha, beta, and gamma subunits of ribosomal eukaryotic elongation factor 1B (eEF1B). Partial dissociation of the complex suggested that the S-transferase activity was associated with the gamma subunit. Moreover, Cibacron blue was found to be a tight binding inhibitor and reactive blue 4 an irreversible time-dependent inhibitor that covalently modified only the gamma subunit. The rate of inactivation by reactive blue 4 was increased more than 600-fold in the presence of trypanothione, and Cibacron blue protected the enzyme from inactivation by 1-chloro-2,4-dinitrobenzene, confirming that these dyes interact with the active site region. Two eEF1Bgamma genes were cloned from C. fasciculata, but recombinant C. fasciculata eEF1Bgamma had no S-transferase activity, suggesting that eEF1Bgamma is unstable in the absence of the other subunits.
Figures



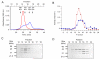
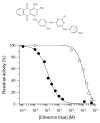

 ) or reactive blue 4 at 2 μM (○), 10 μM (●), 20 μM (□), 50 μM (■), 75 μM (Δ) and 100 μM (▲). (B) Observed rates of TST inactivation by reactive blue 4 as a function of the concentration of inhibitor.
) or reactive blue 4 at 2 μM (○), 10 μM (●), 20 μM (□), 50 μM (■), 75 μM (Δ) and 100 μM (▲). (B) Observed rates of TST inactivation by reactive blue 4 as a function of the concentration of inhibitor.


Similar articles
-
Leishmania major elongation factor 1B complex has trypanothione S-transferase and peroxidase activity.J Biol Chem. 2004 Nov 19;279(47):49003-9. doi: 10.1074/jbc.M407958200. Epub 2004 Aug 18. J Biol Chem. 2004. PMID: 15322082
-
Trypanothione biosynthesis in Leishmania major.Mol Biochem Parasitol. 2005 Jan;139(1):107-16. doi: 10.1016/j.molbiopara.2004.10.004. Mol Biochem Parasitol. 2005. PMID: 15610825
-
Cloning and characterization of the two enzymes responsible for trypanothione biosynthesis in Crithidia fasciculata.J Biol Chem. 1998 Jul 31;273(31):19383-90. doi: 10.1074/jbc.273.31.19383. J Biol Chem. 1998. PMID: 9677355
-
The Achilles' heel of trypanosomatids: trypanothione-mediated hydroperoxide metabolism.Biofactors. 1998;8(1-2):87-91. doi: 10.1002/biof.5520080115. Biofactors. 1998. PMID: 9699014 Review. No abstract available.
-
Targeting trypanothione metabolism in trypanosomatid human parasites.Curr Drug Targets. 2010 Dec;11(12):1614-30. doi: 10.2174/1389450111009011614. Curr Drug Targets. 2010. PMID: 20735352 Review.
Cited by
-
Molecular mechanisms of thermal resistance of the insect trypanosomatid Crithidia thermophila.PLoS One. 2017 Mar 22;12(3):e0174165. doi: 10.1371/journal.pone.0174165. eCollection 2017. PLoS One. 2017. PMID: 28328988 Free PMC article.
-
ATP-dependent ligases in trypanothione biosynthesis--kinetics of catalysis and inhibition by phosphinic acid pseudopeptides.FEBS J. 2008 Nov;275(21):5408-21. doi: 10.1111/j.1742-4658.2008.06670.x. FEBS J. 2008. PMID: 18959765 Free PMC article.
-
Use of antimony in the treatment of leishmaniasis: current status and future directions.Mol Biol Int. 2011;2011:571242. doi: 10.4061/2011/571242. Epub 2011 Jun 8. Mol Biol Int. 2011. PMID: 22091408 Free PMC article.
-
Design, synthesis and biological evaluation of novel inhibitors of Trypanosoma brucei pteridine reductase 1.ChemMedChem. 2011 Feb 7;6(2):302-8. doi: 10.1002/cmdc.201000450. Epub 2010 Dec 29. ChemMedChem. 2011. PMID: 21275054 Free PMC article.
-
Trypanosoma brucei pteridine reductase 1 is essential for survival in vitro and for virulence in mice.Mol Microbiol. 2010 Aug;77(3):658-71. doi: 10.1111/j.1365-2958.2010.07236.x. Epub 2010 Jun 1. Mol Microbiol. 2010. PMID: 20545846 Free PMC article.
References
-
- Fairlamb AH. Trends Parasitol. 2003;19:488–494. - PubMed
-
- Croft SL, Coombs GH. Trends Parasitol. 2003;19:502–508. - PubMed
-
- Zilberstein D, Ephros M. Clinical and laboratory aspects of Leishmania chemotherapy in the era of drug resistance. In: Black SJ, Seed JR, editors. World Class Parasites. Vol. 4. Leishmania Kluwer Academic Press; London: 2002.
-
- Fairlamb AH, Blackburn P, Ulrich P, Chait BT, Cerami A. Science. 1985;227:1485–1487. - PubMed
-
- Fairlamb AH, Cerami A. Annu.Rev.Microbiol. 1992;46:695–729. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

