Filtration rate dependence of hyaluronan reflection by joint-to-lymph barrier: evidence for concentration polarisation
- PMID: 15073278
- PMCID: PMC1665164
- DOI: 10.1113/jphysiol.2004.063529
Filtration rate dependence of hyaluronan reflection by joint-to-lymph barrier: evidence for concentration polarisation
Abstract
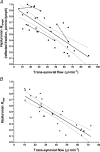
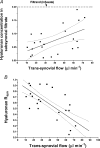
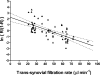
Hyaluronan (HA), a component of synovial fluid, buffers fluid loss from joints. To explain this, a quantitative theory for HA concentration polarisation at a partially sieving synovial lining was developed. The theory predicts a fall in HA reflected fraction R with increased filtration rate. To test this, knees of anaesthetised rabbits were infused with HA and fluorescein-dextran (FD) at constant trans-synovial filtration rates of 6-89 microl min(-1). Samples of femoral lymph, mixed intra-articular fluid and subsynovial fluid after >/= 3 h were analysed by high-performance liquid chromatography. R was calculated as (1 - downstream/upstream concentration), using [FD] to adjust for joint lymph dilution in femoral lymph. Intra-articular HA concentration after >/= 3 h, 0.47 +/- 0.02 mg ml(-1) (mean +/-s.e.m., n= 31), exceeded the infusate concentration, 0.20 mg ml(-1), while subsynovial and lymph [HA] were reduced relative to [FD]. The changes in [HA] demonstrated synovial molecular sieving of HA. R from cavity to lymph (R(lymph)) fell monotonically from 0.93 at 6 microl min(-1) to 0.14 at 89 microl min(-1) (P < 0.0001, regression analysis, n= 33). R values calculated from the intra-articular HA accumulation (R(asp)) or the low subsynovial concentrations (R(syn)) were similar negative functions of filtration rate. R for lymphatic capillary endothelium (R(endo)), calculated from lymph/subsynovial concentration ratios, was effectively zero (-0.03 +/- 0.18, n= 21), confirming that synovium, not initial lymphatic endothelium, is the reflection site. Logarithmic linearisation of the results evaluated the synovial HA reflection coefficient as 0.91. In conclusion, the existence of concentration polarisation during joint fluid drainage was supported by the demonstration of a negative relation between filtration rate and R(lymph), R(asp) and R(syn).
Figures




References
-
- Asari A, Miyauchi S, Matsuzaka S, Ito T, Kominami E, Uchiyama Y. Molecular weight-dependent effects of hyaluronate on the arthritic synovium. Arch Histol Cytol. 1998;61:125–135. - PubMed
-
- Barry SI, Gowman LM, Ethier CR. Obtaining the concentration-dependent diffusion coefficient from ultrafiltration experiments: application to hyaluronate. Biopolymers. 1996;39:1–11.
-
- Blatt WF, Dravid A, Michaels AS, Nelsen L. Solute polarization and cake formation in membrane ultrafiltration: causes, consequences and control techniques. In: Flinn JE, editor. Membrane Science and Technology. New York: Plenum Press; 1970. pp. 47–95.
-
- Brown TJ, Alcorn D, Fraser JRE. Absorption of hyaluronan applied to the surface of intact skin. J Invest Dermatol. 1999;113:740–746. - PubMed
-
- Brown TJ, Laurent UBG, Fraser JRE. Turnover of hyaluronan in synovial joints: elimination of labelled hyaluronan from the knee joint of the rabbit. Exp Physiol. 1991;76:125–134. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

