Oncotic pressures opposing filtration across non-fenestrated rat microvessels
- PMID: 15073281
- PMCID: PMC1665140
- DOI: 10.1113/jphysiol.2003.058255
Oncotic pressures opposing filtration across non-fenestrated rat microvessels
Abstract
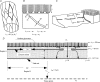
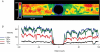
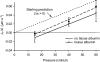
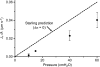
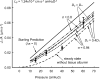
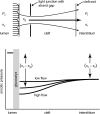
We hypothesized that ultrafiltrate crossing the luminal endothelial glycocalyx through infrequent discontinuities (gaps) in the tight junction (TJ) strand of endothelial clefts reduces albumin diffusive flux from tissue into the 'protected region' of the cleft on the luminal side of the TJ. Thus, the effective oncotic pressure difference (sigma black triangle down pi) opposing filtration is greater than that measured between lumen and interstitial fluid. To test this we measured sigma black triangle down pi across rat mesenteric microvessels perfused with albumin (50 mg ml(-1)) with and without interstitial albumin at the same concentration within a few micrometres of the endothelium as demonstrated by confocal microscopy. We found sigma black triangle down pi was near 70% of luminal oncotic pressure when the tissue concentration equalled that in the lumen. We determined size and frequency of TJ strand gaps in endothelial clefts using serial section electron microscopy. We found nine gaps in the reconstructed clefts having mean spacing of 3.59 microm and mean length of 315 nm. The mean depth of the TJ strand near gaps was 67 nm and the mean cleft path length from lumen to interstitium was 411 nm. With these parameters our three-dimensional hydrodynamic model confirmed that fluid velocity was high at gaps in the TJ strand so that even at relatively low hydraulic pressures the albumin concentration on the tissue side of the glycocalyx was significantly lower than in the interstitium. The results conform to the hypothesis that colloid osmotic forces opposing filtration across non-fenestrated continuous capillaries are developed across the endothelial glycocalyx and that the oncotic pressure of interstitial fluid does not directly determine fluid balance across microvascular endothelium.
Figures






Comment in
-
Revision of the Starling principle: new views of tissue fluid balance.J Physiol. 2004 Jun 15;557(Pt 3):704. doi: 10.1113/jphysiol.2004.066118. Epub 2004 May 6. J Physiol. 2004. PMID: 15131237 Free PMC article. No abstract available.
References
-
- Adamson RH, Lenz JF, Curry FE. Quantitative laser scanning confocal microscopy on single capillaries: permeability measurement. Microcirculation. 1994;1:251–265. - PubMed
-
- Aukland K, Reed RK. Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev. 1993;73:1–78. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

