Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa
- PMID: 15075265
- PMCID: PMC387641
- DOI: 10.1128/EC.3.2.348-358.2004
Role of a mitogen-activated protein kinase pathway during conidial germination and hyphal fusion in Neurospora crassa
Abstract
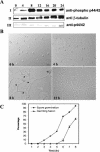
Mitogen-activated protein (MAP) kinase signaling pathways are ubiquitous and evolutionarily conserved in eukaryotic organisms. MAP kinase pathways are composed of a MAP kinase, a MAP kinase kinase, and a MAP kinase kinase kinase; activation is regulated by sequential phosphorylation. Components of three MAP kinase pathways have been identified by genome sequence analysis in the filamentous fungus Neurospora crassa. One of the predicted MAP kinases in N. crassa, MAK-2, shows similarity to Fus3p and Kss1p of Saccharomyces cerevisiae, which are involved in sexual reproduction and filamentation, respectively. In this study, we show that an N. crassa mutant disrupted in mak-2 exhibits a pleiotropic phenotype: derepressed conidiation, shortened aerial hyphae, lack of vegetative hyphal fusion, female sterility, and autonomous ascospore lethality. We assessed the phosphorylation of MAK-2 during conidial germination and early colony development. Peak levels of MAK-2 phosphorylation were most closely associated with germ tube elongation, branching, and hyphal fusion events between conidial germlings. A MAP kinase kinase kinase (NRC-1) is the predicted product of N. crassa nrc-1 locus and is a homologue of STE11 in S. cerevisiae. An nrc-1 mutant shares many of the same phenotypic traits as the mak-2 mutant and, in particular, is a hyphal fusion mutant. We show that MAK-2 phosphorylation during early colony development is dependent upon the presence of NRC-1 and postulate that phosphorylation of MAK-2 is required for hyphal fusion events that occur during conidial germination.
Figures

Similar articles
-
HAM-5 functions as a MAP kinase scaffold during cell fusion in Neurospora crassa.PLoS Genet. 2014 Nov 20;10(11):e1004783. doi: 10.1371/journal.pgen.1004783. eCollection 2014 Nov. PLoS Genet. 2014. PMID: 25412208 Free PMC article.
-
The isolation and characterization of nrc-1 and nrc-2, two genes encoding protein kinases that control growth and development in Neurospora crassa.Genetics. 1998 May;149(1):117-30. doi: 10.1093/genetics/149.1.117. Genetics. 1998. PMID: 9584090 Free PMC article.
-
The nuclear Dbf2-related kinase COT1 and the mitogen-activated protein kinases MAK1 and MAK2 genetically interact to regulate filamentous growth, hyphal fusion and sexual development in Neurospora crassa.Genetics. 2008 Jul;179(3):1313-25. doi: 10.1534/genetics.108.089425. Epub 2008 Jun 18. Genetics. 2008. PMID: 18562669 Free PMC article.
-
Cell fusion in the filamentous fungus, Neurospora crassa.Methods Mol Biol. 2008;475:21-38. doi: 10.1007/978-1-59745-250-2_2. Methods Mol Biol. 2008. PMID: 18979236 Review.
-
Structural and functional organization of growing tips of Neurospora crassa Hyphae.Biochemistry (Mosc). 2014 Jul;79(7):593-607. doi: 10.1134/S0006297914070025. Biochemistry (Mosc). 2014. PMID: 25108323 Review.
Cited by
-
Coordinated Regulation of Protoperithecium Development by MAP Kinases MAK-1 and MAK-2 in Neurospora crassa.Front Microbiol. 2021 Nov 26;12:769615. doi: 10.3389/fmicb.2021.769615. eCollection 2021. Front Microbiol. 2021. PMID: 34899653 Free PMC article.
-
The Saccharomyces cerevisiae PRM1 homolog in Neurospora crassa is involved in vegetative and sexual cell fusion events but also has postfertilization functions.Genetics. 2009 Feb;181(2):497-510. doi: 10.1534/genetics.108.096149. Epub 2008 Dec 8. Genetics. 2009. PMID: 19064710 Free PMC article.
-
The Colletotrichum graminicola striatin orthologue Str1 is necessary for anastomosis and is a virulence factor.Mol Plant Pathol. 2016 Aug;17(6):931-42. doi: 10.1111/mpp.12339. Epub 2016 Feb 18. Mol Plant Pathol. 2016. PMID: 26576029 Free PMC article.
-
Transcriptome analysis implicates secondary metabolite production, redox reactions, and programmed cell death during allorecognition in Cryphonectria parasitica.G3 (Bethesda). 2021 Jan 18;11(1):jkaa021. doi: 10.1093/g3journal/jkaa021. G3 (Bethesda). 2021. PMID: 33561228 Free PMC article.
-
The information highways of a biotechnological workhorse--signal transduction in Hypocrea jecorina.BMC Genomics. 2008 Sep 20;9:430. doi: 10.1186/1471-2164-9-430. BMC Genomics. 2008. PMID: 18803869 Free PMC article.
References
-
- Atienza, J. M., M. Suh, I. Xenarios, R. Landgraf, and J. Colicelli. 2000. Human ERK1 induces filamentous growth and cell wall remodeling pathways in Saccharomyces cerevisiae. J. Biol. Chem. 275:20638-20646. - PubMed
-
- Bistis, G. N. 1981. Chemotropic interactions between trichogynes and conidia of opposite mating-type in Neurospora crassa. Mycologia 73:959-975.
-
- Bobrowicz, P., R. Pawlak, A. Correa, D. Bell-Pedersen, and D. J. Ebbole. 2002. The Neurospora crassa pheromone precursor genes are regulated by the mating type locus and the circadian clock. Mol. Microbiol. 45:795-804. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

