Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1
- PMID: 15075367
- PMCID: PMC452566
- DOI: 10.1091/mbc.e03-12-0888
Role of the midbody matrix in cytokinesis: RNAi and genetic rescue analysis of the mammalian motor protein CHO1
Abstract
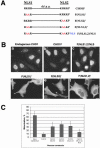
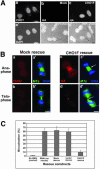
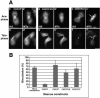
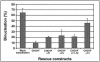
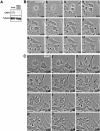
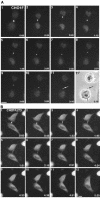
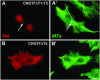
CHO1 is a kinesin-like motor protein essential for cytokinesis in mammalian cells. To analyze how CHO1 functions, we established RNAi and genetic rescue assays. CHO1-depleted cells reached a late stage of cytokinesis but fused back to form binucleate cells because of the absence of the midbody matrix in the middle of the intercellular bridge. Expression of exogenous CHO1 restored the formation of the midbody matrix and rescued cytokinesis in siRNA-treated cells. By analyzing phenotypes rescued with different constructs, it was shown that both motor and stalk domains function in midbody formation, whereas the tail is essential for completion of cytokinesis after the midbody matrix has formed. During the terminal stage of cytokinesis, different subregions of the tail play distinctive roles in stabilizing the midbody matrix and maintaining an association between the midbody and cell cortex. These results demonstrate that CHO1 consists of functionally differentiated subregions that act in concert to ensure complete cell separation.
Figures



References
-
- Boman, A.L., and Kahn, R.A. (1995). Arf proteins: the membrane traffic police? Trends Biochem. Sci. 20, 147-150. - PubMed
-
- Boman, A.L., J. Kuai, J., Zhu, X., Chen, J., Kuriyama, R., and Kahn, R.A. (1999). Arf proteins bind to mitotic kinesin-like protein 1 (MKLP1) in a GTP-dependent fashion. Cell Motil. Cytoskel. 44, 119-132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

