The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts
- PMID: 15075372
- PMCID: PMC420119
- DOI: 10.1091/mbc.e03-08-0613
The adenomatous polyposis coli protein is required for the formation of robust spindles formed in CSF Xenopus extracts
Abstract
Mutations in the adenomatous polyposis coli (APC) protein occur early in colon cancer and correlate with chromosomal instability. Here, we show that depletion of APC from cystostatic factor (CSF) Xenopus extracts leads to a decrease in microtubule density and changes in tubulin distribution in spindles and asters formed in such extracts. Addition of full-length APC protein or a large, N-terminally truncated APC fragment to APC-depleted extracts restored normal spindle morphology and the intact microtubule-binding site of APC was necessary for this rescue. These data indicate that the APC protein plays a role in the formation of spindles that is dependent on its effect on microtubules. Spindles formed in cycled extracts were not sensitive to APC depletion. In CSF extracts, spindles predominantly formed from aster-like intermediates, whereas in cycled extracts chromatin was the major site of initial microtubule polymerization. These data suggest that APC is important for centrosomally driven spindle formation, which was confirmed by our finding that APC depletion reduced the size of asters nucleated from isolated centrosomes. We propose that lack of microtubule binding in cancer-associated mutations of APC may contribute to defects in the assembly of mitotic spindles and lead to missegregation of chromosomes.
Figures

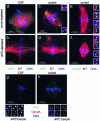
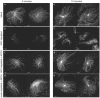
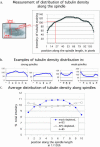
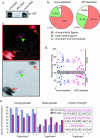
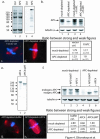



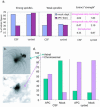
References
-
- Bienz, M., and Clevers, H. (2000). Linking colorectal cancer to Wnt signaling. Cell 103, 311-320. - PubMed
-
- Boland, C.R., and Kim, Y.S. (1993). Gastrointestinal polyposis syndromes. In: Gastrointestinal Disease, ed. M.H. Sleisinger and J.S. Fordtran, Philadelphia: WB Saunders, 1430-1436.
-
- Carazo-Salas, R.E., and Karsenti, E. (2003). Long-range communication between chromatin and microtubules in Xenopus egg extracts. Curr. Biol. 13, 1728-1733. - PubMed
-
- Deka, J., Kuhlmann, J., and Muller, O. (1998). A domain within the tumor suppressor protein APC shows very similar biochemical properties as the microtubule-associated protein tau. Eur. J. Biochem. 253, 591-597. - PubMed
-
- Desai, A., Murray, A.W., Mitchison, T.J., and Walczak, C.E. (1999). The use of Xenopus egg extracts to study mitotic spindle assembly and function in vitro. Methods Cell Biol. 61, 385-412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

