Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA
- PMID: 15075376
- PMCID: PMC420116
- DOI: 10.1091/mbc.e03-10-0745
Vascular endothelial-cadherin regulates cytoskeletal tension, cell spreading, and focal adhesions by stimulating RhoA
Abstract
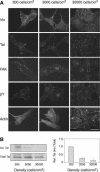
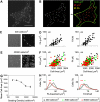
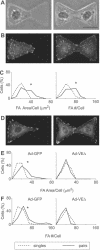
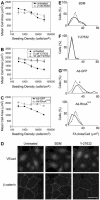
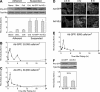
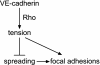
Changes in vascular endothelial (VE)-cadherin-mediated cell-cell adhesion and integrin-mediated cell-matrix adhesion coordinate to affect the physical and mechanical rearrangements of the endothelium, although the mechanisms for such cross talk remain undefined. Herein, we describe the regulation of focal adhesion formation and cytoskeletal tension by intercellular VE-cadherin engagement, and the molecular mechanism by which this occurs. Increasing the density of endothelial cells to increase cell-cell contact decreased focal adhesions by decreasing cell spreading. This contact inhibition of cell spreading was blocked by disrupting VE-cadherin engagement with an adenovirus encoding dominant negative VE-cadherin. When changes in cell spreading were prevented by culturing cells on a micropatterned substrate, VE-cadherin-mediated cell-cell contact paradoxically increased focal adhesion formation. We show that VE-cadherin engagement mediates each of these effects by inducing both a transient and sustained activation of RhoA. Both the increase and decrease in cell-matrix adhesion were blocked by disrupting intracellular tension and signaling through the Rho-ROCK pathway. In all, these findings demonstrate that VE-cadherin signals through RhoA and the actin cytoskeleton to cross talk with cell-matrix adhesion and thereby define a novel pathway by which cell-cell contact alters the global mechanical and functional state of cells.
Figures

References
-
- Abercrombie, M., and Turner, A.A. (1978). Contract reactions influencing cell locomotion of a mouse sarcoma in culture. Med. Biol. 56, 299-303. - PubMed
-
- Amano, M., Fukata, Y., and Kaibuchi, K. (2000). Regulation and functions of Rho-associated kinase. Exp. Cell Res. 261, 44-51. - PubMed
-
- Anastasiadis, P.Z., and Reynolds, A.B. (2001). Regulation of Rho GTPases by p120-catenin. Curr. Opin. Cell Biol. 13, 604-610. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

