BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways
- PMID: 15075392
- PMCID: PMC395917
- DOI: 10.1073/pnas.0401367101
BMP4 supports self-renewal of embryonic stem cells by inhibiting mitogen-activated protein kinase pathways
Abstract
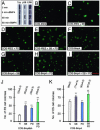
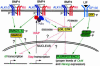
The fate of pluripotent stem cells is tightly controlled during early embryonic development. Both the derivation and the maintenance of embryonic stem cells (ES cells) in vitro depend on feeder cell-derived growth factors that are largely unidentified. To dissect the mechanisms governing pluripotency, we conducted a screen to identify factors that are produced by mouse embryonic fibroblast STO cells and are required to maintain the pluripotency of ES cells. One of the factors is bone morphogenetic protein 4 (BMP4). Unexpectedly, the major effect of BMP4 on the self-renewal of ES cells is accomplished by means of the inhibition of both extracellular receptor kinase (ERK) and p38 mitogen-activated protein kinase (MAPK) pathways, and inhibitors of ERK and p38 MAPKs mimic the effect of BMP4 on ES cells. Importantly, inhibition of the p38 MAPK pathway by SB203580 overcomes the block in deriving ES cells from blastocysts lacking a functional Alk3, the BMP type IA receptor. These results uncover a paradigm for BMP signaling in the biology of pluripotent stem cells.
Figures



References
-
- Evans, M. J. & Kaufman, M. H. (1981) Nature 292, 154-156. - PubMed
-
- Bradley, A., Evans, M., Kaufman, M. H. & Robertson, E. (1984) Nature 309, 255-256. - PubMed
-
- Nichols, J., Zevnik, B., Anastassiadis, K., Niwa, H., Klewe-Nebenius, D., Chambers, I., Scholer, H. & Smith, A. (1998) Cell 95, 379-391. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

