Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites
- PMID: 15077203
- PMCID: PMC1181981
- DOI: 10.1086/420771
Polymorphisms in the low-density lipoprotein receptor-related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites
Abstract
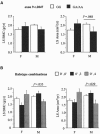
Stature, bone size, and bone mass are interrelated traits with high heritability, but the major genes that govern these phenotypes remain unknown. Independent genomewide quantitative-trait locus studies have suggested a locus for bone-mineral density and stature at chromosome 11q12-13, a region harboring the low-density lipoprotein receptor-related protein 5 (LRP5) gene. Mutations in the LRP5 gene were recently implicated in osteoporosis-pseudoglioma and "high-bone-mass" syndromes. To test whether polymorphisms in the LRP5 gene contribute to bone-mass determination in the general population, we studied a cross-sectional cohort of 889 healthy whites of both sexes. Significant associations were found for a missense substitution in exon 9 (c.2047G-->A) with lumbar spine (LS)-bone-mineral content (BMC) (P=.0032), with bone area (P=.0014), and with stature (P=.0062). The associations were observed mainly in adult men, in whom LRP5 polymorphisms accounted for <or=15% of the traits' variances. Results of haplotype analysis of five single-nucleotide polymorphisms in the LRP5 region suggest that additional genetic variation within the locus might also contribute to bone-mass and size determination. To confirm our results, we investigated whether LRP5 haplotypes were associated with 1-year gain in vertebral bone mass and size in 386 prepubertal children. Significant associations were observed for changes in BMC (P=.0348) and bone area (P=.0286) in males but not females, independently supporting our observations of a mostly male-specific effect, as seen in the adults. Together, these results suggest that LRP5 variants significantly contribute to LS-bone-mass and size determination in men by influencing vertebral bone growth during childhood.
Figures
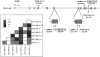


References
Electronic-Database Information
-
- dbSNP Database, http://www.ncbi.nlm.nih.gov/SNP/ (for SNPs IVS4-4T→C [accession number rs314776], exon 9 c.1980 G→A [accession number rs2277268], exon 9 c.2047 G→A [accession number rs4988321], exon 10 c.2268 C→T [accession number rs2306862], IVS10 +6T→C [accession number rs4988322], exon 15 c.3405 A→G [accession number rs556442], exon 18 c.4037 C→T [accession number rs3736228], and exon 19 c.4137 C→T [accession number rs3736229])
-
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for OPPG and HBM)
References
-
- Bonjour JP, Theintz G, Buchs B, Slosman D, Rizzoli R (1991) Critical years and stages of puberty for spinal and femoral bone mass accumulation during adolescence. J Clin Endocrinol Metab 73:555–563 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

