Molecular recognition at purine and pyrimidine nucleotide (P2) receptors
- PMID: 15078212
- PMCID: PMC4428617
- DOI: 10.2174/1568026043450961
Molecular recognition at purine and pyrimidine nucleotide (P2) receptors
Abstract
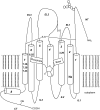
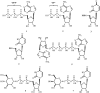
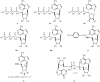
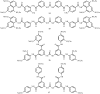
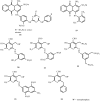
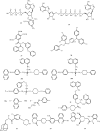
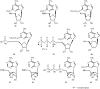
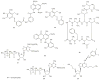
In comparison to other classes of cell surface receptors, the medicinal chemistry at P2X (ligand-gated ion channels) and P2Y (G protein-coupled) nucleotide receptors has been relatively slow to develop. Recent effort to design selective agonists and antagonists based on a combination of library screening, empirical modification of known ligands, and rational design have led to the introduction of potent antagonists of the P2X(1) (derivatives of pyridoxal phosphates and suramin), P2X(3)(A-317491), P2X(7) (derivatives of the isoquinoline KN-62), P2Y(1)(nucleotide analogues MRS 2179 and MRS 2279), P2Y(2)(thiouracil derivatives such as AR-C126313), and P2Y(12)(nucleotide/nucleoside analogues AR-C69931X and AZD6140) receptors. A variety of native agonist ligands (ATP, ADP, UTP, UDP, and UDP-glucose) are currently the subject of structural modification efforts to improve selectivity. MRS2365 is a selective agonist for P2Y(1)receptors. The dinucleotide INS 37217 potently activates the P2Y(2)receptor. UTP-gamma-S and UDP-beta-S are selective agonists for P2Y(2)/P2Y(4)and P2Y(6)receptors, respectively. The current knowledge of the structures of P2X and P2Y receptors, is derived mainly from mutagenesis studies. Site-directed mutagenesis has shown that ligand recognition in the human P2Y(1)receptor involves individual residues of both the TMs (3, 5, 6, and 7), as well as EL 2 and 3. The binding of the negatively-charged phosphate moiety is dependent on positively charged lysine and arginine residues near the exofacial side of TMs 3 and 7.
Figures

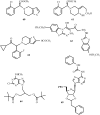
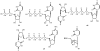
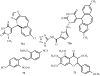
References
-
- Burnstock G. Introduction: P2 Receptors. This volume. - PubMed
-
- Abbracchio MP, Burnstock G. Purinergic signalling: pathophysiological roles. Jpn J Pharmacol. 1998;78:113–145. - PubMed
-
- Müller C. P2-Pyrimidinergic receptors and their ligands. Curr Pharm Des. 2002;8:2353–69. - PubMed
-
- Zimmermann H, Braun N. Ecto-nucleotidases—molecular structures, catalytic properties, and functional roles in the nervous system. Prog Brain Res. 1999;120:371–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

