Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1
- PMID: 15078816
- PMCID: PMC395851
- DOI: 10.1101/gad.1189604
Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1
Abstract
Two evolutionarily distant plant species, rice (Oryza sativa L.), a short-day (SD) plant, and Arabidopsis thaliana, a long-day plant, share a conserved genetic network controlling photoperiodic flowering. The orthologous floral regulators-rice Heading date 1 (Hd1) and Arabidopsis CONSTANS (CO)-integrate circadian clock and external light signals into mRNA expression of the FLOWERING LOCUS T (FT) group floral inducer. Here, we report that the rice Early heading date 1 (Ehd1) gene, which confers SD promotion of flowering in the absence of a functional allele of Hd1, encodes a B-type response regulator that might not have an ortholog in the Arabidopsis genome. Ehd1 mRNA was induced by 1-wk SD treatment, and Ehd1 may promote flowering by inducing FT-like gene expression only under SD conditions. Microarray analysis further revealed a few MADS box genes downstream of Ehd1. Our results indicate that a novel two-component signaling cascade is integrated into the conserved pathway in the photoperiodic control of flowering in rice.
Figures

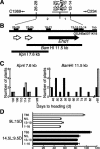

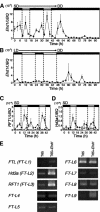

References
-
- Burge, C. and Karlin, S. 1997. Prediction of complete gene structures in human genomic DNA. J. Mol. Biol. 268: 78–94. - PubMed
-
- Chang, C. and Stadler, R. 2001. Ethylene hormone receptor action in Arabidopsis. BioEssays 23: 619–627. - PubMed
-
- Chung, Y.Y., Kim, S.R., Finkel, D., Yanofsky, M.F., and An, G. 1994. Early flowering and reduced apical dominance result from extopic expression of a rice MADS box gene. Plant Mol. Biol. 26: 657–665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
