p27Kip1 modulates cell migration through the regulation of RhoA activation
- PMID: 15078817
- PMCID: PMC395846
- DOI: 10.1101/gad.1185504
p27Kip1 modulates cell migration through the regulation of RhoA activation
Abstract
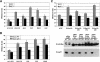
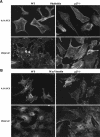
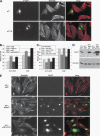
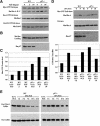
The tumor suppressor p27(Kip1) is an inhibitor of cyclin/cyclin-dependent kinase (CDK) complexes and plays a crucial role in cell cycle regulation. However, p27(Kip1) also has cell cycle-independent functions. Indeed, we find that p27(Kip1) regulates cell migration, as p27(Kip1)-null fibroblasts exhibit a dramatic decrease in motility compared with wild-type cells. The regulation of motility by p27(Kip1) is independent of its cell-cycle regulatory functions, as re-expression of both wild-type p27(Kip1) and a mutant p27(Kip1) (p27CK(-)) that cannot bind to cyclins and CDKs rescues migration of p27(-/-) cells. p27(-/-) cells have increased numbers of actin stress fibers and focal adhesions. This is reminiscent of cells in which the Rho pathway is activated. Indeed, active RhoA levels were increased in cells lacking p27(Kip1). Moreover, inhibition of ROCK, a downstream effector of Rho, was able to rescue the migration defect of p27(-/-) cells in response to growth factors. Finally, we found that p27(Kip1) binds to RhoA, thereby inhibiting RhoA activation by interfering with the interaction between RhoA and its activators, the guanine-nucleotide exchange factors (GEFs). Together, the data suggest a novel role for p27(Kip1) in regulating cell migration via modulation of the Rho pathway.
Figures



References
-
- Amano, A., Chihara, K., Kimura, K., Fukata, Y., Nakamura, N., Matsuura, Y., and Kaibuchi, K. 1997. Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science 275: 1308–1311. - PubMed
-
- Anayama, T., Furihata, M., Ishikawa, T., Ohtsuki, Y., and Ogoshi, S. 1998. Positive correlation between p27Kip1 expression and progression in human esophageal squamous cell carcinoma. Int. J. Cancer 79: 439–443. - PubMed
-
- Arthur, W.T., Petch, L.A., and Burridge, K. 2000. Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Curr. Biol. 10: 719–722. - PubMed
-
- Bar-Sagi, D. and Hall, A. 2000. Ras and Rho GTPases: A family reunion. Cell 103: 227–238. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
