Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue
- PMID: 15078900
- PMCID: PMC2211899
- DOI: 10.1084/jem.20022212
Blockade of attachment and fusion receptors inhibits HIV-1 infection of human cervical tissue
Abstract
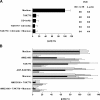
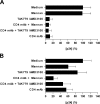
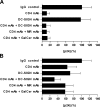
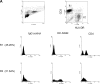
Identification of cellular factors involved in HIV-1 entry and transmission at mucosal surfaces is critical for understanding viral pathogenesis and development of effective prevention strategies. Here we describe the evaluation of HIV-1 entry inhibitors for their ability to prevent infection of, and dissemination from, human cervical tissue ex vivo. Blockade of CD4 alone or CCR5 and CXCR4 together inhibited localized mucosal infection. However, simultaneous blockade of CD4 and mannose-binding C-type lectin receptors including dendritic cell-specific intercellular adhesion molecule-grabbing integrin was required to inhibit HIV-1 uptake and dissemination by migratory cells. In contrast, direct targeting of HIV-1 by neutralizing mAb b12 and CD4-IgG2 (PRO-542) blocked both localized infection and viral dissemination pathways. Flow cytometric analysis and immunostaining of migratory cells revealed two major populations, CD3(+)HLA-DR(-) and CD3(-)HLA-DR(+) cells, with a significant proportion of the latter also expressing dendritic cell-specific intercellular adhesion molecule-grabbing integrin. Bead depletion studies demonstrated that such HLA-DR(+) cells accounted for as much as 90% of HIV-1 dissemination. Additional studies using immature monocyte-derived dendritic cells demonstrated that although mannose-binding C-type lectin receptors and CD4 are the principal receptors for gp120, other mechanisms may account for virus capture. Our identification of the predominant receptors involved in HIV-1 infection and dissemination within human cervical tissue highlight important targets for microbicide development.
Figures




Comment in
-
HIV transmission: closing all the doors.J Exp Med. 2004 Apr 19;199(8):1037-40. doi: 10.1084/jem.20040426. Epub 2004 Apr 12. J Exp Med. 2004. PMID: 15078894 Free PMC article. Review. No abstract available.
References
-
- Shattock, R.J., and J.P. Moore. 2003. Inhibiting sexual transmission of HIV-1 infection. Nat. Rev. Microbiol. 1:25–34. - PubMed
-
- Miller, C.J., and R.J. Shattock. 2003. Target cells in vaginal HIV transmission. Microbes Infect. 5:59–67. - PubMed
-
- Collins, K.B., B.K. Patterson, G.J. Naus, D.V. Landers, and P. Gupta. 2000. Development of an in vitro organ culture model to study transmission of HIV-1 in the female genital tract. Nat. Med. 6:475–479. - PubMed
-
- Coombs, R.W., P.S. Reichelderfer, and A.L. Landay. 2003. Recent observations on HIV type-1 infection in the genital tract of men and women. AIDS. 17:455–480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

