Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor
- PMID: 15078903
- PMCID: PMC2172094
- DOI: 10.1083/jcb.200312055
Role of the mammalian retromer in sorting of the cation-independent mannose 6-phosphate receptor
Abstract
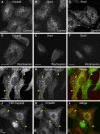
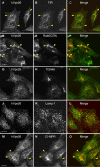
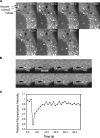
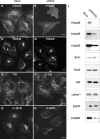
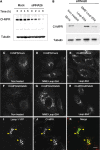
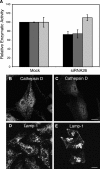
The cation-independent mannose 6-phosphate receptor (CI-MPR) mediates sorting of lysosomal hydrolase precursors from the TGN to endosomes. After releasing the hydrolase precursors into the endosomal lumen, the unoccupied receptor returns to the TGN for further rounds of sorting. Here, we show that the mammalian retromer complex participates in this retrieval pathway. The hVps35 subunit of retromer interacts with the cytosolic domain of the CI-MPR. This interaction probably occurs in an endosomal compartment, where most of the retromer is localized. In particular, retromer is associated with tubular-vesicular profiles that emanate from early endosomes or from intermediates in the maturation from early to late endosomes. Depletion of retromer by RNA interference increases the lysosomal turnover of the CI-MPR, decreases cellular levels of lysosomal hydrolases, and causes swelling of lysosomes. These observations indicate that retromer prevents the delivery of the CI-MPR to lysosomes, probably by sequestration into endosome-derived tubules from where the receptor returns to the TGN.
Figures
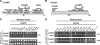


References
-
- Chen, H.J., J. Remmler, J.C. Delaney, D.J. Messner, and P. Lobel. 1993. Mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor. A consensus casein kinase II site followed by 2 leucines near the carboxyl terminus is important for intracellular targeting of lysosomal enzymes. J. Biol. Chem. 268:22338–22346. - PubMed
-
- Chen, H.J., J. Yuan, and P. Lobel. 1997. Systematic mutational analysis of the cation-independent mannose 6-phosphate/insulin-like growth factor II receptor cytoplasmic domain. An acidic cluster containing a key aspartate is important for function in lysosomal enzyme sorting. J. Biol. Chem. 272:7003–7012. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

