The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3
- PMID: 15078926
- PMCID: PMC387694
- DOI: 10.1128/jvi.78.9.4454-4462.2004
The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3
Abstract
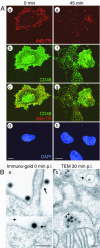
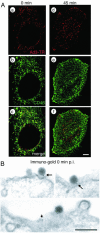
Many human adenovirus (Ad) serotypes use the coxsackie B virus-Ad receptor (CAR). Recently, CD46 was suggested to be a receptor of species B Ad serotype 11 (Ad11), Ad14, Ad16, Ad21, Ad35, and Ad50. Using Sindbis virus-mediated cDNA library expression, we identify here the membrane cofactor protein CD46 as a surface receptor of species B Ad3. All four major CD46 transcripts and one minor CD46 transcript expressed in nucleated human cells were isolated. Rodent BHK cells stably expressing the BC1 form of CD46 bound radiolabeled Ad3 with a dissociation constant of 0.3 nM, identical to that of CD46-positive HeLa cells expressing twice as many Ad3 binding sites. Pull-down experiments with recombinant Ad3 fibers and a soluble form of the CD46 extracellular domain linked to the Fc portion of human immunoglobulin G (CD46ex-Fc) indicated direct interactions of the Ad3 fiber knob with CD46ex-Fc but not CARex-Fc (Fc-linked extracellular domain of CAR). Ad3 colocalized with cell surface CD46 in both rodent and human cells at the light and electron microscopy levels. Anti-CD46 antibodies and CD46ex-Fc inhibited Ad3 binding to CD46-expressing BHK cells more than 10-fold and to human cells 2-fold. In CD46-expressing BHK cells, wild-type Ad3 and a chimeric Ad consisting of the Ad5 capsid and the Ad3 fiber elicited dose-dependent cytopathic effects and transgene expression, albeit less efficiently than in human cells. Together, our results show that all of the major splice forms of CD46 are predominant and functional binding sites of Ad3 on CD46-expressing rodent and human cells but may not be the sole receptor of species B Ads on human cells. These results have implications for understanding viral pathogenesis and therapeutic gene delivery.
Figures

References
-
- Adrian, T., G. Wadell, J. C. Hierholzer, and R. Wigand. 1986. DNA restriction analysis of adenovirus prototypes 1 to 41. Arch. Virol. 91:277-290. - PubMed
-
- Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. - PubMed
-
- Chroboczek, J., R. W. H. Ruigrok, and S. Cusack. 1995. Adenovirus fiber, vol. I. Springer-Verlag KG, Berlin, Germany. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

