CCAAT/enhancer binding protein alpha binds to the Epstein-Barr virus (EBV) ZTA protein through oligomeric interactions and contributes to cooperative transcriptional activation of the ZTA promoter through direct binding to the ZII and ZIIIB motifs during induction of the EBV lytic cycle
- PMID: 15078966
- PMCID: PMC387681
- DOI: 10.1128/jvi.78.9.4847-4865.2004
CCAAT/enhancer binding protein alpha binds to the Epstein-Barr virus (EBV) ZTA protein through oligomeric interactions and contributes to cooperative transcriptional activation of the ZTA promoter through direct binding to the ZII and ZIIIB motifs during induction of the EBV lytic cycle
Abstract
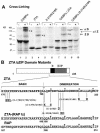
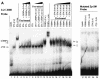
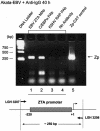
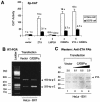
The Epstein-Barr virus (EBV)-encoded ZTA protein interacts strongly with and stabilizes the cellular CCAAT/enhancer binding protein alpha (C/EBPalpha), leading to the induction of p21-mediated G(1) cell cycle arrest. Despite the strong interaction between these two basic leucine zipper (bZIP) family proteins, the ZTA and C/EBPalpha subunits do not heterodimerize, as indicated by an in vitro cross-linking assay with in vitro-cotranslated (35)S-labeled C/EBPalpha and (35)S-labeled ZTA protein. Instead, they evidently form a higher-order oligomeric complex that competes with C/EBPalpha binding but not with ZTA binding in electrophoretic mobility shift assays (EMSAs). Glutathione S-transferase affinity assays with mutant ZTA proteins revealed that the basic DNA binding domain and the key leucine zipper residues required for homodimerization are all required for the interaction with C/EBPalpha. ZTA is known to bind to two ZRE sites within the ZTA promoter and to positively autoregulate its own expression in transient cotransfection assays, but there is conflicting evidence about whether it does so in vivo. Examination of the proximal ZTA upstream promoter region by in vitro EMSA analysis revealed two high-affinity C/EBP binding sites (C-2 and C-3), which overlap the ZII and ZIIIB motifs, implicated as playing a key role in lytic cycle induction. A chromatin immunoprecipitation assay confirmed the in vivo binding of both endogenous C/EBPalpha and ZTA protein to the ZTA promoter after lytic cycle induction but not during the latent state in EBV-infected Akata cells. Reporter assays revealed that cotransfected C/EBPalpha activated the ZTA promoter even more effectively than cotransfected ZTA. However, synergistic activation of the ZTA promoter was not observed when ZTA and C/EBPalpha were cotransfected together in either HeLa or DG75 cells. Mutagenesis of either the ZII or the ZIIIB sites in the ZTA promoter strongly reduced C/EBPalpha transactivation, suggesting that these sites act cooperatively. Furthermore, the introduction of exogenous C/EBPalpha into EBV-infected HeLa-BX1 cells induced endogenous ZTA mRNA and protein expression, as demonstrated by both reverse transcription-PCR and immunoblotting assays. Finally, double-label immunofluorescence assays suggested that EAD protein expression was activated even better than ZTA expression in latently infected C/EBPalpha-transfected Akata cells, perhaps because of the presence of a strong B-cell-specific repressed chromatin conformation on the ZTA promoter itself during EBV latency.
Figures








References
-
- Bauer, G., P. Hofler, and H. Zur Hausen. 1982. Epstein-Barr virus induction by a serum factor. I. Induction and cooperation with additional inducers. Virology 121:184-194. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

