Transduction of terminally differentiated neurons by avian sarcoma virus
- PMID: 15078971
- PMCID: PMC387698
- DOI: 10.1128/jvi.78.9.4902-4906.2004
Transduction of terminally differentiated neurons by avian sarcoma virus
Abstract
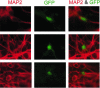
Recent studies have demonstrated that avian sarcoma virus (ASV) can transduce cycle-arrested cells. Here, we have assessed quantitatively the transduction efficiency of an ASV vector in naturally arrested mouse hippocampal neurons. This efficiency was determined by comparing the number of transduced cells after infection of differentiated neurons versus dividing progenitor cells. The results indicate that ASV is able to transduce these differentiated neurons efficiently and that this activity is not the result of infection of residual dividing cells. The transduction efficiency of the ASV vector was found to be intermediate between the relatively high and low efficiencies obtained with human immunodeficiency virus type 1 and murine leukemia virus vectors, respectively.
Figures




References
-
- Chen, W., X. Wu, D. N. Levasseur, H. Liu, L. Lai, J. C. Kappes, and T. M. Townes. 2000. Lentiviral vector transduction of hematopoietic stem cells that mediate long-term reconstitution of lethally irradiated mice. Stem Cells 18:352-359. - PubMed
-
- Deglon, N., and P. Aebischer. 2002. Lentiviruses as vectors for CNS diseases. Curr. Top. Microbiol. Immunol. 261:191-209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

