Tobacco mosaic virus infection spreads cell to cell as intact replication complexes
- PMID: 15079061
- PMCID: PMC395962
- DOI: 10.1073/pnas.0401221101
Tobacco mosaic virus infection spreads cell to cell as intact replication complexes
Abstract
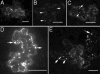
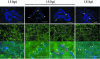
Plant viruses encode movement proteins (MPs) that facilitate cell-cell transport of infection through plasmodesmata. Intracellular and intercellular spread of virus replication complexes (VRCs) of tobacco mosaic virus was followed in intact leaf tissue from 12 to 36 h post infection (hpi) by using confocal microscopy. From 12 hpi, VRCs in primary infected cells were associated with cortical endoplasmic reticulum, and at 14 hpi, exhibited high intracellular mobility ( approximately 160 nm/sec); mobility was slowed between 14 and 16 hpi ( approximately 40 nm/sec), and by 18 hpi, VRCs were stationary, adjacent to plasmodesmata. VRCs traversed the plasmodesmata between 18 and 20 hpi. The process of formation and movement of VRCs was repeated in adjacent cells in 3-4 h vs. 20 h from primary infected cells. The rapid intracellular movement of the VRCs and the spread to adjacent cells was blocked by inhibitors of filamentous actin and myosin, but not by inhibitors of microtubules. We propose a model whereby cell-cell spread of tobamovirus infection is accomplished by subviral replication complexes that initiate TMV replication immediately after entry to adjacent cells.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

