A possible therapeutic target for Lou Gehrig's disease
- PMID: 15079068
- PMCID: PMC395856
- DOI: 10.1073/pnas.0401934101
A possible therapeutic target for Lou Gehrig's disease
Figures
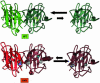
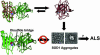
Comment on
-
Dimer destabilization in superoxide dismutase may result in disease-causing properties: structures of motor neuron disease mutants.Proc Natl Acad Sci U S A. 2004 Apr 20;101(16):5976-81. doi: 10.1073/pnas.0305143101. Epub 2004 Mar 31. Proc Natl Acad Sci U S A. 2004. PMID: 15056757 Free PMC article.
References
-
- Julien, J. P. (2001) Cell 104, 581–591. - PubMed
-
- Caughey, B. & Lansbury, P. T., Jr. (2003) Annu. Rev. Neurosci. 26, 267–298. - PubMed
-
- Ray, S. S., Nowak, R. J., Strokovich, K., Brown, R. H., Jr., Walz, T. & Lansbury, P. T., Jr. (2004) Biochemistry 39, in press. - PubMed
-
- Siddique, T., Nijhawan, D. & Hentati, A. (1996) Neurology 47, S27–S35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

