Dendritic cells cross-present HIV antigens from live as well as apoptotic infected CD4+ T lymphocytes
- PMID: 15079077
- PMCID: PMC395928
- DOI: 10.1073/pnas.0304860101
Dendritic cells cross-present HIV antigens from live as well as apoptotic infected CD4+ T lymphocytes
Abstract
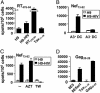
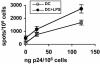
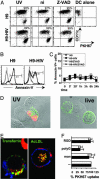
A better understanding of the antigen presentation pathways that lead to CD8(+) T cell recognition of HIV epitopes in vivo is needed to achieve better immune control of HIV replication. Here, we show that cross-presentation of very small amounts of HIV proteins from apoptotic infected CD4(+) T lymphocytes by dendritic cells to CD8(+) T cells is much more efficient than other known HIV presentation pathways, i.e., direct presentation of infectious virus or cross-presentation of defective virus. Unexpectedly, dendritic cells also take up actively antigens into endosomes from live infected CD4(+) T lymphocytes and cross-present them as efficiently as antigens derived from apoptotic infected cells. Moreover, live infected CD4(+) T cells costimulate cross-presenting dendritic cells in the process. Therefore, dendritic cells can present very small amounts of viral proteins from infected T cells either after apoptosis, which is frequent during HIV infection, or not. Thus, if HIV expression is transiently induced while costimulation is enhanced (for instance after IL-2 and IFNalpha immune therapy), this HIV antigen presentation pathway could be exploited to eradicate latently infected reservoirs, which are poorly recognized by patients' immune systems.
Figures
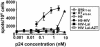
References
-
- Letvin, N. L. & Walker, B. D. (2003) Nat. Med. 9, 861-866. - PubMed
-
- Wilson, J. D., Ogg, G. S., Allen, R. L., Davis, C., Shaunak, S., Downie, J., Dyer, W., Workman, C., Sullivan, S., McMichael, A. J. & Rowland-Jones, S. L. (2000) AIDS 14, 225-233. - PubMed
-
- Blankson, J. N., Persaud, D. & Siliciano, R. F. (2002) Annu. Rev. Med. 53, 557-593. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

