Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop
- PMID: 15079078
- PMCID: PMC395897
- DOI: 10.1073/pnas.0400891101
Molecular basis for the inhibition of the carboxyltransferase domain of acetyl-coenzyme-A carboxylase by haloxyfop and diclofop
Abstract
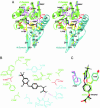
Acetyl-CoA carboxylases (ACCs) are crucial for the metabolism of fatty acids, making these enzymes important targets for the development of therapeutics against obesity, diabetes, and other diseases. The carboxyltransferase (CT) domain of ACC is the site of action of commercial herbicides, such as haloxyfop, diclofop, and sethoxydim. We have determined the crystal structures at up to 2.5-A resolution of the CT domain of yeast ACC in complex with the herbicide haloxyfop or diclofop. The inhibitors are bound in the active site, at the interface of the dimer of the CT domain. Unexpectedly, inhibitor binding requires large conformational changes for several residues in this interface, which create a highly conserved hydrophobic pocket that extends deeply into the core of the dimer. Two residues that affect herbicide sensitivity are located in this binding site, and mutation of these residues disrupts the structure of the domain. Other residues in the binding site are strictly conserved among the CT domains.
Figures
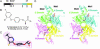
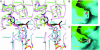
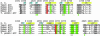
References
-
- Friedman, J. M. (2003) Science 299, 856–858. - PubMed
-
- Hill, J. O., Wyatt, H. R., Reed, G. W. & Peters, J. C. (2003) Science 299, 853–855. - PubMed
-
- Pi-Sunyer, X. (2003) Science 299, 859–860. - PubMed
-
- Alberts, A. W. & Vagelos, P. R. (1972) in The Enzymes, ed. Boyer, P. D. (Academic, New York), Vol. 6, pp. 37–82.
-
- Wakil, S. J., Stoops, J. K. & Joshi, V. C. (1983) Annu. Rev. Biochem. 52, 537–579. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

