Effects of charge state and cationizing agent on the electron capture dissociation of a peptide
- PMID: 15080732
- PMCID: PMC1343469
- DOI: 10.1021/ac035431p
Effects of charge state and cationizing agent on the electron capture dissociation of a peptide
Abstract
Electron capture dissociation (ECD) is a promising method for de novo sequencing proteins and peptides and for locating the positions of labile posttranslational modifications and binding sites of noncovalently bound species. We report the ECD of a synthetic peptide containing 10 alanine residues and 6 lysine residues uniformly distributed across the sequence. ECD of the (M + 2H)(2+) produces a limited range of c (c(7)-c(15)) and z (z(9)-z(15)) fragment ions, but ECD of higher charge states produces a wider range of c (c(2)-c(15)) and z (z(2)-z(6), z(9)-z(15)) ions. Fragmentation efficiency increases with increasing precursor charge state, and efficiencies up to 88% are achieved. Heating the (M + 2H)(2+) to 150 degrees C does not increase the observed range of ECD fragment ions, indicating that the limited products are due to backbone cleavages occurring near charges and not due to effects of tertiary structure. ECD of the (M + 2Li)(2+) and (M + 2Cs)(2+) produces di- and monometalated analogues of the same c and z ions observed from the (M + 2H)(2+), with the abundance of dimetalated fragment ions increasing with fragment ion mass, a result consistent with the metal cations being located near the peptide termini to minimize Coulombic repulsion. In stark contrast to the ECD results, collisional activation of cesiated dications overwhelmingly results in ejection of Cs(+). The abundance of cesiated fragment ions formed from ECD of the (M + Cs + Li)(2+) exceeds that of lithiated fragment ions by 10:1. ECD of the (M + H + Li)(2+) results in exclusively lithiated c and z ions, indicating an overwhelming preference for neutralization and cleavage at protonated sites over metalated sites. These results are consistent with preferential neutralization of the cation with the highest recombination energy.
Figures


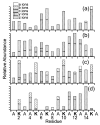


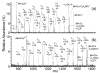




References
-
- Agarwal KL, Johnstone RA, Kenner GW, Millington DS, Sheppard RC. Nature. 1968;219:498–499. - PubMed
-
- McLafferty FW, Venkataraghavan R, Irving P. Biochem Biophys Res Commun. 1970;39:274–278. - PubMed
-
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Science. 1989;246:64–71. - PubMed
-
- Hillenkamp F, Karas M, Beavis RC, Chait BT. Anal Chem. 1991;63:1193A–1202A. - PubMed
-
- Siuzdak G, Bothner B, Yeager M, Brugidou C, Fauquet CM, Hoey K, Chang CM. Chem Biol. 1996;3:45–48. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

