Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis
- PMID: 15082468
- PMCID: PMC1755088
- DOI: 10.1136/ard.2003.020313
Patient Preference for Placebo, Acetaminophen (paracetamol) or Celecoxib Efficacy Studies (PACES): two randomised, double blind, placebo controlled, crossover clinical trials in patients with knee or hip osteoarthritis
Abstract
Background: Acetaminophen (paracetamol) is recommended as the initial pharmacological treatment for knee or hip osteoarthritis. However, survey and clinical trial data indicate greater efficacy for non-steroidal anti-inflammatory drugs and cyclo-oxygenase-2 specific inhibitors.
Design: Two randomised, double blind, placebo controlled, crossover multicentre clinical trials, Patient Preference for Placebo, Acetaminophen or Celecoxib Efficacy Studies (PACES).
Patients: Osteoarthritis of knee or hip.
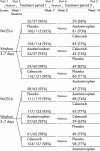
Intervention: "Wash out" of treatment; randomisation; 6 weeks of celecoxib 200 mg/day, acetaminophen 1000 mg four times a day, or placebo; second "wash out;" crossover to 6 weeks of second treatment.
Measurements: Western Ontario McMaster Osteoarthritis Index (WOMAC), visual analogue pain scale, patient preference between two treatments.
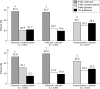
Results: Celecoxib was more efficacious than acetaminophen in both periods in both studies; WOMAC and pain scale scores differed at p<0.05 in period II and both periods combined of PACES-a and in periods I and II and both periods combined in PACES-b, but not in period I of PACES-a. Acetaminophen was more efficacious than placebo, generally p<0.05 in PACES-b, and >0.05 in PACES-a. Patient preferences were 53% celecoxib v 24% acetaminophen in PACES-a (p<0.001) and 50% v 32% in PACES-b (p = 0.009); 37% acetaminophen v 28% placebo in PACES-a (p = 0.340) and 48% v 24% in PACES-b (p = 0.007). No clinically or statistically significant differences were seen in adverse events or tolerability among the three treatment groups.
Conclusions: Greater efficacy was seen for celecoxib v acetaminophen v placebo, while adverse events and tolerability were similar. Variation in results and statistical significance in the two different trials are of interest.
Figures

Comment in
-
A historic issue of the Annals: three papers examine paracetamol in osteoarthritis.Ann Rheum Dis. 2004 Aug;63(8):897-900. doi: 10.1136/ard.2004.020727. Ann Rheum Dis. 2004. PMID: 15249315 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

