Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed
- PMID: 15082480
- PMCID: PMC1755008
- DOI: 10.1136/ard.2003.013052
Efficacy and safety of adalimumab as monotherapy in patients with rheumatoid arthritis for whom previous disease modifying antirheumatic drug treatment has failed
Abstract
Objective: To evaluate the efficacy and safety of monotherapy with adalimumab in patients with RA for whom previous DMARD treatment has failed.
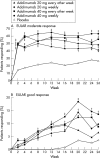
Methods: In a 26 week, double blind, placebo controlled, phase III trial, 544 patients with RA were randomised to monotherapy with adalimumab 20 mg every other week, 20 mg weekly, 40 mg every other week, 40 mg weekly, or placebo. The primary efficacy end point was > or =20% improvement in the ACR core criteria (ACR20 response). Secondary efficacy end points included ACR50, ACR70, EULAR responses, and the Disability Index of the Health Assessment Questionnaire (HAQ DI).
Results: After 26 weeks, patients treated with adalimumab 20 mg every other week, 20 mg weekly, 40 mg every other week, and 40 mg weekly had significantly better response rates than those treated with placebo: ACR20 (35.8%, 39.3%, 46.0%, 53.4%, respectively v 19.1%; p< or =0.01); ACR50 (18.9%, 20.5%, 22.1%, 35.0% v 8.2%; p< or =0.05); ACR70 (8.5%, 9.8%, 12.4%, 18.4% v 1.8%; p< or =0.05). Moderate EULAR response rates were significantly greater with adalimumab than with placebo (41.5%, 48.2%, 55.8%, 63.1% v 26.4%; p< or =0.05). Patients treated with adalimumab achieved better improvements in mean HAQ DI than those receiving placebo (-0.29, -0.39, -0.38, -0.49 v -0.07; p< or =0.01). No significant differences were found between adalimumab and placebo treated patients for serious adverse events, serious infections, or malignancies. Injection site reaction occurred in 10.6% and 0.9% of adalimumab and placebo treated patients, respectively (p< or =0.05).
Conclusion: Among patients with RA for whom previous DMARD treatment had failed, adalimumab monotherapy achieved significant, rapid, and sustained improvements in disease activity and improved physical function and was safe and well tolerated.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

