Oats to children with newly diagnosed coeliac disease: a randomised double blind study
- PMID: 15082581
- PMCID: PMC1774046
- DOI: 10.1136/gut.2003.026948
Oats to children with newly diagnosed coeliac disease: a randomised double blind study
Abstract
Background: Treatment of coeliac disease (CD) requires lifelong adherence to a strict gluten free diet (GFD) which hitherto has consisted of a diet free of wheat, rye, barley, and oats. Recent studies, mainly in adults, have shown that oats are non-toxic to CD patients. In children, only open studies comprising a small number of patients have been performed.
Aim: To determine if children with CD tolerate oats in their GFD.
Patients and methods: In this double blind multicentre study involving eight paediatric clinics, 116 children with newly diagnosed CD were randomised to one of two groups: one group was given a standard GFD (GFD-std) and one group was given a GFD with additional wheat free oat products (GFD-oats). The study period was one year. Small bowel biopsy was performed at the beginning and end of the study. Serum IgA antigliadin, antiendomysium, and antitissue transglutaminase antibodies were monitored at 0, 3, 6, and 12 months.
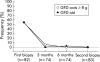
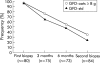
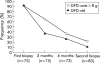
Results: Ninety three patients completed the study. Median (range) daily oat intake in the GFD-oats group (n = 42) was 15 (5-40) g at the six month control and 15 (0-43) g at the end of the study. All patients were in clinical remission after the study period. The GFD-oats and GFD-std groups did not differ significantly at the end of the study regarding coeliac serology markers or small bowel mucosal architecture, including numbers of intraepithelial lymphocytes. Significantly more children in the youngest age group withdrew.
Conclusions: This is the first randomised double blind study showing that the addition of moderate amounts of oats to a GFD does not prevent clinical or small bowel mucosal healing, or humoral immunological downregulation in coeliac children. This is in accordance with the findings of studies in adult coeliacs and indicates that oats, added to the otherwise GFD, can be accepted and tolerated by the majority of children with CD.
Figures

References
-
- Dicke WK. Coeliakie, MD Thesis Utrecht: University of Utrecht, 1950.
-
- Wieser H. Prolamins in cereals. In: Lohiniemi S, Collin P, Mäki M, eds. Changing features of coeliac disease. Tampere: The Finnish Coeliac Society, 1998:25–30.
-
- Shan L, Molberg O, Parrot I, et al. Structural basis for gluten intolerance in celiac sprue. Science 2002;297:2275–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
