Adapter protein SH2-B beta undergoes nucleocytoplasmic shuttling: implications for nerve growth factor induction of neuronal differentiation
- PMID: 15082760
- PMCID: PMC387738
- DOI: 10.1128/MCB.24.9.3633-3647.2004
Adapter protein SH2-B beta undergoes nucleocytoplasmic shuttling: implications for nerve growth factor induction of neuronal differentiation
Abstract
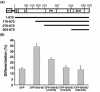
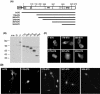
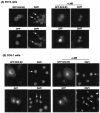
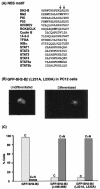
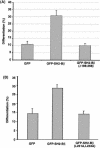
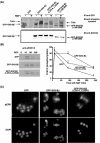
The adapter protein SH2-B has been shown to bind to activated nerve growth factor (NGF) receptor TrkA and has been implicated in NGF-induced neuronal differentiation and the survival of sympathetic neurons. However, the mechanism by which SH2-B enhances and maintains neurite outgrowth is unclear. We examined the ability of truncation mutants to regulate neuronal differentiation and observed that certain truncation mutants localized in the nucleus rather than in the cytoplasm or at the plasma membrane as reported for wild-type SH2-B beta. Addition of the nuclear export inhibitor leptomycin B caused both overexpressed wild-type and endogenous SH2-B beta to accumulate in the nucleus of both PC12 cells and COS-7 cells as did deletion of a putative nuclear export sequence (amino acids 224 to 233) or mutation of two critical lysines in that sequence. Deleting or mutating the nuclear export signal caused SH2-B beta to lose its ability to enhance NGF-induced differentiation of PC12 cells. Neither the NGF-induced phosphorylation of ERKs 1 and 2 nor their subcellular distribution was altered in PC12 cells stably expressing the nuclear export-defective SH2-B beta(L231A, L233A). These data provide strong evidence that SH2-B beta shuttles constitutively between the nucleus and cytoplasm. However, SH2-B beta needs continuous access to the cytoplasm and/or plasma membrane to participate in NGF-induced neurite outgrowth. These data also suggest that the stimulatory effect of SH2-B beta on NGF-induced neurite outgrowth of PC12 cells is either downstream of ERKs or via some other pathway yet to be identified.
Figures



Similar articles
-
SH2B1beta (SH2-Bbeta) enhances expression of a subset of nerve growth factor-regulated genes important for neuronal differentiation including genes encoding urokinase plasminogen activator receptor and matrix metalloproteinase 3/10.Mol Endocrinol. 2008 Feb;22(2):454-76. doi: 10.1210/me.2007-0384. Epub 2007 Oct 18. Mol Endocrinol. 2008. PMID: 17947375 Free PMC article.
-
Nucleocytoplasmic shuttling of the adapter protein SH2B1beta (SH2-Bbeta) is required for nerve growth factor (NGF)-dependent neurite outgrowth and enhancement of expression of a subset of NGF-responsive genes.Mol Endocrinol. 2009 Jul;23(7):1077-91. doi: 10.1210/me.2009-0011. Epub 2009 Apr 16. Mol Endocrinol. 2009. PMID: 19372237 Free PMC article.
-
SH2-B is required for nerve growth factor-induced neuronal differentiation.J Biol Chem. 1999 Apr 9;274(15):10590-4. doi: 10.1074/jbc.274.15.10590. J Biol Chem. 1999. PMID: 10187854
-
SH2-B is a positive regulator of nerve growth factor-mediated activation of the Akt/Forkhead pathway in PC12 cells.J Biol Chem. 2004 Jan 2;279(1):133-41. doi: 10.1074/jbc.M310040200. Epub 2003 Oct 16. J Biol Chem. 2004. PMID: 14565960
-
Molecular dissection of TrkA signal transduction pathways mediating differentiation in human neuroblastoma cells.Oncogene. 2000 Apr 13;19(16):2043-51. doi: 10.1038/sj.onc.1203518. Oncogene. 2000. PMID: 10803465
Cited by
-
Identification of SH2B1β as a focal adhesion protein that regulates focal adhesion size and number.J Cell Sci. 2011 Sep 15;124(Pt 18):3095-105. doi: 10.1242/jcs.081547. Epub 2011 Aug 30. J Cell Sci. 2011. PMID: 21878491 Free PMC article.
-
SH2B1beta (SH2-Bbeta) enhances expression of a subset of nerve growth factor-regulated genes important for neuronal differentiation including genes encoding urokinase plasminogen activator receptor and matrix metalloproteinase 3/10.Mol Endocrinol. 2008 Feb;22(2):454-76. doi: 10.1210/me.2007-0384. Epub 2007 Oct 18. Mol Endocrinol. 2008. PMID: 17947375 Free PMC article.
-
Crucial Role of the SH2B1 PH Domain for the Control of Energy Balance.Diabetes. 2019 Nov;68(11):2049-2062. doi: 10.2337/db19-0608. Epub 2019 Aug 22. Diabetes. 2019. PMID: 31439647 Free PMC article.
-
Identification of βIIΣ1-Spectrin as a Binding Partner of the GH-regulated Human Obesity Scaffold Protein SH2B1.Endocrinology. 2025 Feb 5;166(3):bqaf003. doi: 10.1210/endocr/bqaf003. Endocrinology. 2025. PMID: 39801013
-
SH2B1β interacts with STAT3 and enhances fibroblast growth factor 1-induced gene expression during neuronal differentiation.Mol Cell Biol. 2014 Mar;34(6):1003-19. doi: 10.1128/MCB.00940-13. Epub 2014 Jan 6. Mol Cell Biol. 2014. PMID: 24396070 Free PMC article.
References
-
- Cheng, S., M. S. Geddis, and V. Rehder. 2002. Local calcium changes regulate the length of growth cone filopodia. J. Neurobiol. 50:263-275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
