Human enhancer of invasion-cluster, a coiled-coil protein required for passage through mitosis
- PMID: 15082789
- PMCID: PMC387757
- DOI: 10.1128/MCB.24.9.3957-3971.2004
Human enhancer of invasion-cluster, a coiled-coil protein required for passage through mitosis
Abstract
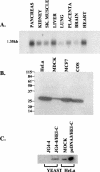
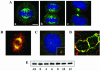
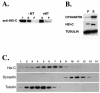
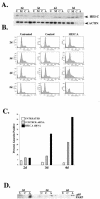
In a cross-species overexpression approach, we used the pseudohyphal transition of Saccharomyces cerevisiae as a model screening system to identify human genes that regulate cell morphology and the cell cycle. Human enhancer of invasion-cluster (HEI-C), encoding a novel evolutionarily conserved coiled-coil protein, was isolated in a screen for human genes that induce agar invasion in S. cerevisiae. In human cells, HEI-C is primarily localized to the spindle during mitosis. Depletion of HEI-C in vivo with short interfering RNAs results in severe mitotic defects. Analysis by immunofluorescence, flow cytometry analysis, and videomicroscopy indicates that HEI-C-depleted cells form metaphase plates with normal timing after G(2)/M transition, although in many cases cells have disorganized mitotic spindles. Subsequently, severe defects occur at the metaphase-anaphase transition, characterized by a significant delay at this stage or, more commonly, cellular disintegration accompanied by the display of classic biochemical markers of apoptosis. These mitotic defects occur in spite of the fact that HEI-C-depleted cells retain functional cell cycle checkpoints, as these cells arrest normally following nocodazole or hydroxyurea treatment. These results place HEI-C as a novel regulator of spindle function and integrity during the metaphase-anaphase transition.
Figures




References
-
- Amon, A. 1999. The spindle checkpoint. Curr. Opin. Genet. Dev. 9:69-75. - PubMed
-
- Barral, Y., S. Jentsch, and C. Mann. 1995. G1 cyclin turnover and nutrient uptake are controlled by a common pathway in yeast. Genes Dev. 9:399-409. - PubMed
-
- Braverman, L. E., and L. A. Quilliam. 1999. Identification of Grb4/Nckbeta, a src homology 2 and 3 domain-containing adapter protein having similar binding and biological properties to Nck. J. Biol. Chem. 274:5542-5549. - PubMed
-
- Chan, G. K., and T. J. Yen. 2003. The mitotic checkpoint: a signaling pathway that allows a single unattached kinetochore to inhibit mitotic exit. Prog. Cell Cycle Res. 5:431-439. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
