Altered localization of retinoid X receptor alpha coincides with loss of retinoid responsiveness in human breast cancer MDA-MB-231 cells
- PMID: 15082790
- PMCID: PMC387734
- DOI: 10.1128/MCB.24.9.3972-3982.2004
Altered localization of retinoid X receptor alpha coincides with loss of retinoid responsiveness in human breast cancer MDA-MB-231 cells
Abstract
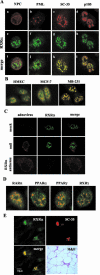
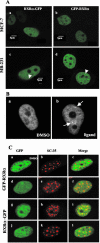
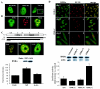
To understand the mechanism of retinoid resistance, we studied the subcellular localization and function of retinoid receptors in human breast cancer cell lines. Retinoid X receptor alpha (RXR alpha) localized throughout the nucleoplasm in retinoid-sensitive normal human mammary epithelial cells and in retinoid-responsive breast cancer cell line (MCF-7), whereas it was found in the splicing factor compartment (SFC) of the retinoid-resistant MDA-MB-231 breast cancer cell line and in human breast carcinoma tissue. In MDA-MB-231 cells, RXR alpha was not associated with active transcription site in the presence of ligand. Similarly, ligand-dependent RXR homo- or heterodimer-mediated transactivation on RXR response element or RARE showed minimal response to ligand in MDA-MB-231 cells. Infecting MDA-MB-231 cells with adenoviral RXR alpha induced nucleoplasmic overexpression of RXR alpha and resulted in apoptosis upon treatment with an RXR ligand. This suggests that nucleoplasmic RXR alpha restores retinoid sensitivity. Epitope-tagged RXR alpha and a C-terminus deletion mutant failed to localize to the SFC. Moreover, RXR alpha localization to the SFC was inhibited with RXR alpha C-terminus peptide. This peptide also induced ligand-dependent transactivation on RXRE. Therefore, the RXR alpha C terminus may play a role in the intranuclear localization of RXR alpha. Our results provide evidence that altered localization of RXR alpha to the SFC may be an important factor for the loss of retinoid responsiveness in MDA-MB-231 breast cancer cells.
Figures


Similar articles
-
Inhibition of trans-retinoic acid-resistant human breast cancer cell growth by retinoid X receptor-selective retinoids.Mol Cell Biol. 1997 Nov;17(11):6598-608. doi: 10.1128/MCB.17.11.6598. Mol Cell Biol. 1997. PMID: 9343423 Free PMC article.
-
A retinoid X receptor (RXR)-selective retinoid reveals that RXR-alpha is potentially a therapeutic target in breast cancer cell lines, and that it potentiates antiproliferative and apoptotic responses to peroxisome proliferator-activated receptor ligands.Breast Cancer Res. 2004;6(5):R546-55. doi: 10.1186/bcr913. Epub 2004 Jul 23. Breast Cancer Res. 2004. PMID: 15318936 Free PMC article.
-
Retinoic acid receptor alpha expression correlates with retinoid-induced growth inhibition of human breast cancer cells regardless of estrogen receptor status.Cancer Res. 1997 Jul 1;57(13):2642-50. Cancer Res. 1997. PMID: 9205071
-
Retinoid X receptor (RXR) within the RXR-retinoic acid receptor heterodimer binds its ligand and enhances retinoid-dependent gene expression.Mol Cell Biol. 1997 Feb;17(2):644-55. doi: 10.1128/MCB.17.2.644. Mol Cell Biol. 1997. PMID: 9001218 Free PMC article.
-
Linkage between retinoid and fatty acid receptors: implications for breast cancer prevention.Eur J Cancer Prev. 2002 Aug;11(4):319-25. doi: 10.1097/00008469-200208000-00002. Eur J Cancer Prev. 2002. PMID: 12195157 Review.
Cited by
-
The effect of body weight on altered expression of nuclear receptors and cyclooxygenase-2 in human colorectal cancers.Nutr J. 2007 Sep 3;6:20. doi: 10.1186/1475-2891-6-20. Nutr J. 2007. PMID: 17767717 Free PMC article.
-
Regulation of the nongenomic actions of retinoid X receptor-α by targeting the coregulator-binding sites.Acta Pharmacol Sin. 2015 Jan;36(1):102-12. doi: 10.1038/aps.2014.109. Epub 2014 Dec 1. Acta Pharmacol Sin. 2015. PMID: 25434990 Free PMC article. Review.
-
AEG-1 regulates retinoid X receptor and inhibits retinoid signaling.Cancer Res. 2014 Aug 15;74(16):4364-77. doi: 10.1158/0008-5472.CAN-14-0421. Cancer Res. 2014. PMID: 25125681 Free PMC article.
-
Cytoplasmic Localization of RXRα Determines Outcome in Breast Cancer.Cancers (Basel). 2021 Jul 26;13(15):3756. doi: 10.3390/cancers13153756. Cancers (Basel). 2021. PMID: 34359656 Free PMC article.
-
Correlation of thyroid hormone, retinoid X, peroxisome proliferator-activated, vitamin D and oestrogen/progesterone receptors in breast carcinoma.Oncol Lett. 2012 Oct;4(4):665-671. doi: 10.3892/ol.2012.799. Epub 2012 Jul 13. Oncol Lett. 2012. PMID: 23205080 Free PMC article.
References
-
- Bischoff, E. D., M. M. Gottardis, T. E. Moon, R. A. Heyman, and W. W. Lamph. 1998. Beyond tamoxifen: the retinoid X receptor-selective ligand LGD1069 (TARGRETIN) causes complete regression of mammary carcinoma. Cancer Res. 58:479-484. - PubMed
-
- Boccardo, F., L. Canobbio, M. Resasco, A. U. Decensi, G. Pastorino, and F. Brema. 1990. Phase II study of tamoxifen and high-dose retinyl acetate in patients with advanced breast cancer. J. Cancer Res. Clin. Oncol. 116:503-506. - PubMed
-
- Cassidy, J., M. Lippman, A. Lacroix, and G. Peck. 1982. Phase II trial of 13-cis-retinoic acid in metastatic breast cancer. Eur. J. Cancer Clin. Oncol. 18:925-928. - PubMed
-
- Castelein, H., P. E. Declercq, and M. Baes. 1997. DNA binding preferences of PPAR alpha/RXR alpha heterodimers. Biochem. Biophys. Res. Commun. 233:91-95. - PubMed
-
- Chen, A. C., X. Guo, F. Derguini, and L. J. Gudas. 1997. Human breast cancer cells and normal mammary epithelial cells: retinol metabolism and growth inhibition by the retinol metabolite 4-oxoretinol. Cancer Res. 57:4642-4651. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
