Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a
- PMID: 15082829
- PMCID: PMC404117
- DOI: 10.1073/pnas.0308636100
Extracellular acidosis increases neuronal cell calcium by activating acid-sensing ion channel 1a
Abstract
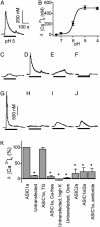
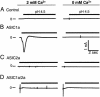
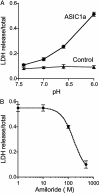
Acid-sensing ion channel (ASIC) 1a subunit is expressed in synapses of central neurons where it contributes to synaptic plasticity. However, whether these channels can conduct Ca(2+) and thereby raise the cytosolic Ca(2+) concentration, [Ca(2+)](c), and possibly alter neuronal physiology has been uncertain. We found that extracellular acidosis opened ASIC1a channels, which provided a pathway for Ca(2+) entry and elevated [Ca(2+)](c) in wild-type, but not ASIC1(-/-), hippocampal neurons. Acid application also raised [Ca(2+)](c) and evoked Ca(2+) currents in heterologous cells expressing ASIC1a. Although ASIC2a is also expressed in central neurons, neither ASIC2a homomultimeric channels nor ASIC1a/2a heteromultimers showed H(+)-activated [Ca(2+)](c) elevation or Ca(2+) currents. Because extracellular acidosis accompanying cerebral ischemia contributes to neuronal injury, we tested the effect of acidosis on cell death measured as lactate dehydrogenase release. Eliminating ASIC1a from neurons or treating ASIC1a-expressing cells with the ASIC blocker amiloride attenuated acidosis-induced cell injury. These results indicate that ASIC1a provides a non-voltage-gated pathway for Ca(2+) to enter neurons. Thus, it may provide a target for modulation of [Ca(2+)](c).
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

