Regulation of glomerular endothelial cell proteoglycans by glucose
- PMID: 15082898
- PMCID: PMC2822306
- DOI: 10.3346/jkms.2004.19.2.245
Regulation of glomerular endothelial cell proteoglycans by glucose
Abstract
The presence of heparan sulfate proteoglycan (HSPG) in anionic sites in the lamina rara interna of glomerular basement membrane suggests that the proteoglycan may be deposited by the glomerular endothelial cells (GEndo). We have previously demonstrated that bovine GEndo in vitro synthesize perlecan, a species of glomerular basement membrane HSPG. In this study we examined whether high glucose medium regulates the GEndo metabolism of glycopeptides including perlecan. Metabolic labeling of glycoconjugates with 35S-SO4, sequential ion exchange and Sepharose CL-4B chromatography of labeled glycoconjugates, and northern analysis were performed. Incubation of GEndo for 8 to 14 weeks (but not for 1-2 weeks) in medium containing 30 mM glucose resulted in nearly 50% reduction in the synthesis of cell layer and medium 35SO4-labeled low anionic glycoproteins and proteoglycans, including that of basement membrane HSPG (Kav 0.42) compared to GEndo grown in 5 mM glucose medium; no changes in anionic charge density or hydrodynamic size of proteoglycans were noted. Northern analysis demonstrated that the mRNA abundance of perlecan was reduced by 47% in cells incubated with 30 mM glucose. Our data suggest that high glucose medium reduces the GEndo synthesis of perlecan by regulating its gene expression. Reduced synthesis of perlecan by GEndo may contribute to proteinuria seen in diabetic nephropathy.
Figures


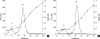

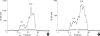

References
-
- Vernier RL, Steffes MW, Sisson-Ross S, Mauer SM. Heparan sulfate proteoglycan in the glomerular basement membrane of type I diabetes mellitus. Kidney Int. 1992;41:1070–1080. - PubMed
-
- Rosenzweig LJ, Kanwar YS. Removal of sulfated (heparan sulfate) and nonsulfated (hyaluronic acid) glycosaminoglycans results in increased permeability of the glomerular basement membrane to 125I-bovine serum albumin. Lab Invest. 1982;47:177–184. - PubMed
-
- Kasinath BS, Kanwar YS. Glomerular Basement Membrane: Biology and Physiology. In: Rohrbach DH, Timpl R, editors. Molecular and Cellular Aspects of Basement Membrane. San Diego: Academic Press; 1993. pp. 89–106.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

