Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure
- PMID: 15082970
- PMCID: PMC1356274
- DOI: 10.1097/01.sla.0000124298.74199.e5
Prospective, randomized, multicenter, controlled trial of a bioartificial liver in treating acute liver failure
Abstract
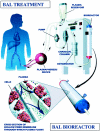
Objective: The HepatAssist liver support system is an extracorporeal porcine hepatocyte-based bioartificial liver (BAL). The safety and efficacy of the BAL were evaluated in a prospective, randomized, controlled, multicenter trial in patients with severe acute liver failure.
Summary background data: In experimental animals with acute liver failure, we demonstrated beneficial effects of the BAL. Similarly, Phase I trials of the BAL in acute liver failure patients yielded promising results.
Methods: A total of 171 patients (86 control and 85 BAL) were enrolled. Patients with fulminant/subfulminant hepatic failure and primary nonfunction following liver transplantation were included. Data were analyzed with and without accounting for the following confounding factors: liver transplantation, time to transplant, disease etiology, disease severity, and treatment site.
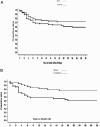
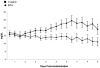
Results: For the entire patient population, survival at 30 days was 71% for BAL versus 62% for control (P = 0.26). After exclusion of primary nonfunction patients, survival was 73% for BAL versus 59% for control (n = 147; P = 0.12). When survival was analyzed accounting for confounding factors, in the entire patient population, there was no difference between the 2 groups (risk ratio = 0.67; P = 0.13). However, survival in fulminant/subfulminant hepatic failure patients was significantly higher in the BAL compared with the control group (risk ratio = 0.56; P = 0.048).
Conclusions: This is the first prospective, randomized, controlled trial of an extracorporeal liver support system, demonstrating safety and improved survival in patients with fulminant/subfulminant hepatic failure.
Figures
References
-
- Kondrup J, Almdal T, Vilstrup H, et al. High volume plasma exchange in fulminant hepatic failure. Int J Artif Organs. 1992;15:669–676. - PubMed
-
- Awad SS, Swaniker F, Magee J, et al. Results of a phase I trial evaluating a liver support device utilizing albumin dialysis. Surgery. 2001;130:354–362. - PubMed
-
- Horslen SP, Hammel JM, Fristoe LW, et al. Extracorporeal liver perfusion using human and pig livers for acute liver failure. Transplantation. 2000;70:1472–1478. - PubMed
-
- Ellis AJ, Hughes RD, Wendon JA, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology. 1996;24:1446–1451. - PubMed
-
- Mullon C, Pitkin Z. The HepatAssist® Bioartificial Liver Support System: clinical study and pig hepatocyte process. Expert Opin Invest Drugs. 1999;8:229–235. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

