A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer
- PMID: 15083178
- PMCID: PMC2409709
- DOI: 10.1038/sj.bjc.6601736
A phase II study of gemcitabine and cisplatin in chemotherapy-naive, unresectable gall bladder cancer
Abstract
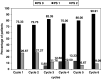
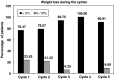
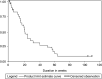
The primary objective of this study was to determine the response rates of the gemcitabine and cisplatin combination in unresectable gall bladder cancer patients. The secondary objectives were to evaluate the toxicity, time to progressive disease, and overall survival. Chemonaïve patients with histologically proven, unresectable bidimensionally measurable gall bladder cancer were enrolled into this study. All patients were required to have a Zubrod's performance status <or=2, no prior radiotherapy, and adequate major organ function. Patients received gemcitabine (1000 mg x m(-2) intravenously over 30-60 min) on days 1 and 8, and cisplatin (70 mg x m(-2) intravenously over 2 h) on day 1, every 21 days. Response assessment was done by a CT scan after every other cycle of chemotherapy. In all, 30 patients were eligible for efficacy and toxicity analysis. There were four (13.3%) complete responders, seven (23.3%) partial responders, and seven (23.3%) with stable disease, with four (13.2%) patients showing disease progression. The median time to progression was 18 weeks (95% confidence interval (CI) 14-24 weeks), and the median duration of response was 13.5 weeks (range 5.5-104 weeks). The median overall survival was 20 weeks (95% CI 14-31 weeks), with 1-year survival rate of 18.6%. WHO grade 3 or 4 anaemia was seen in seven (23.3%) and four (13.3%) patients, respectively. Five (16.6%) patients each experienced grade 3 or 4 neutropenia, and grade 3 or 4 thrombocytopenia was seen in three (10%) and two (6.6%) patients, respectively. The present study shows that gemcitabine/cisplatin combination is well tolerated and active in advanced unresectable gall bladder cancer.
Figures
References
-
- Andre T, Louvet C, Artru P, Selle F, Turnigand C, Garcia ML, Avenin D, Maindrault F, Provent S, DeGramont A (2001) A phase II study of gemcitabine and oxaliplatin (GEMOX) in advanced biliary adenocarcinoma (ABA). Preliminary results. Eur J Cancer 37, (Suppl 6) 19
-
- Arroyo G, Gallardo J, Rubio B, Orlandi L, Yañez M, Gamargo C, Ahumada M, Zarba J, Berlingeri G, Kowalyszyn R (2001) Gemcitabine (GEM) in advanced biliary tract cancer (ABTC). Experience from Chile and Argentina in phase II trials. Proc Am Soc Clin Oncol 20: A626 (abstract)
-
- Carraro S, Servienio PJ, Bruno MF, Castillo Odena MDS, Roca E, Jovtis S, Felci N, Araujo C (2001) Gemcitabine and cisplatin in locally advanced or metastatic gallbladder and bile duct adenocarcinomas. Proc Am Soc Clin Oncol 20: A2333 (abstract)
-
- De Groen PC, Gores GJ, LaRusso NF, Gunderson LL, Nagorney DM (1999) Biliary tract cancers. N Engl J Med 341: 1368–1378 - PubMed
-
- Dobrila R, Kovac D, Depolo A, Dintinjana M (2000) Gemcitabine in patients with nonresectable cancer of the biliary system or advanced gallbladder cancer. Am J Gastroenterol 95: 2476 (abstract)
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical