Molecular analysis of muskelin identifies a conserved discoidin-like domain that contributes to protein self-association
- PMID: 15084145
- PMCID: PMC1133863
- DOI: 10.1042/BJ20040253
Molecular analysis of muskelin identifies a conserved discoidin-like domain that contributes to protein self-association
Abstract
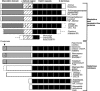
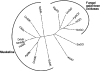
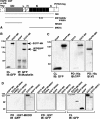
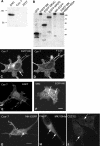
Muskelin is an intracellular protein with a C-terminal kelch-repeat domain that was initially characterized as having functional involvement in cell spreading on the extracellular matrix glycoprotein thrombospondin-1. As one approach to understanding the functional properties of muskelin, we have combined bioinformatic and biochemical studies. Through analysis of a new dataset of eight animal muskelins, we showed that the N-terminal region of the polypeptide corresponds to a predicted discoidin-like domain. This domain architecture is conserved in fungal muskelins and reveals a structural parallel between the muskelins and certain extracellular fungal galactose oxidases, although the phylogeny of the two groups appears distinct. In view of the fact that a number of kelch-repeat proteins have been shown to self-associate, co-immunoprecipitation, protein pull-down assays and studies of cellular localization were carried out with wild-type, deletion mutant and point mutant muskelins to investigate the roles of the discoidin-like and kelch-repeat domains. We obtained evidence for cis- and trans-interactions between the two domains. These studies provide evidence that muskelin self-associates through a head-to-tail mechanism involving the discoidin-like domain.
Figures



Similar articles
-
Structure of mouse muskelin discoidin domain and biochemical characterization of its self-association.Acta Crystallogr D Biol Crystallogr. 2014 Nov;70(Pt 11):2863-74. doi: 10.1107/S139900471401894X. Epub 2014 Oct 16. Acta Crystallogr D Biol Crystallogr. 2014. PMID: 25372678
-
Characterization of a Drosophila melanogaster orthologue of muskelin.Gene. 2002 Sep 4;297(1-2):69-78. doi: 10.1016/s0378-1119(02)00887-9. Gene. 2002. PMID: 12384287
-
Regulation of post-translational modifications of muskelin by protein kinase C.Int J Biochem Cell Biol. 2007;39(2):366-78. doi: 10.1016/j.biocel.2006.09.003. Epub 2006 Sep 19. Int J Biochem Cell Biol. 2007. PMID: 17049906
-
Structural similarities and functional diversity of eukaryotic discoidin-like domains.Biochim Biophys Acta. 2007 Sep;1774(9):1069-78. doi: 10.1016/j.bbapap.2007.07.007. Epub 2007 Jul 24. Biochim Biophys Acta. 2007. PMID: 17702679 Review.
-
The UNC-45 myosin chaperone: from worms to flies to vertebrates.Int Rev Cell Mol Biol. 2014;313:103-44. doi: 10.1016/B978-0-12-800177-6.00004-9. Int Rev Cell Mol Biol. 2014. PMID: 25376491 Free PMC article. Review.
Cited by
-
Myosin motors at neuronal synapses: drivers of membrane transport and actin dynamics.Nat Rev Neurosci. 2013 Apr;14(4):233-47. doi: 10.1038/nrn3445. Epub 2013 Mar 13. Nat Rev Neurosci. 2013. PMID: 23481482 Review.
-
Novel role of the muskelin-RanBP9 complex as a nucleocytoplasmic mediator of cell morphology regulation.J Cell Biol. 2008 Aug 25;182(4):727-39. doi: 10.1083/jcb.200801133. Epub 2008 Aug 18. J Cell Biol. 2008. PMID: 18710924 Free PMC article.
-
Characteristics of the Kelch domain containing (KLHDC) subfamily and relationships with diseases.FEBS Lett. 2025 Apr;599(8):1094-1112. doi: 10.1002/1873-3468.15108. Epub 2025 Jan 30. FEBS Lett. 2025. PMID: 39887712 Free PMC article. Review.
-
Neuronal expression of muskelin in the rodent central nervous system.BMC Neurosci. 2007 May 2;8:28. doi: 10.1186/1471-2202-8-28. BMC Neurosci. 2007. PMID: 17474996 Free PMC article.
-
The Cdk5 activator P39 specifically links muskelin to myosin II and regulates stress fiber formation and actin organization in lens.Exp Cell Res. 2015 Jan 1;330(1):186-98. doi: 10.1016/j.yexcr.2014.08.003. Epub 2014 Aug 13. Exp Cell Res. 2015. PMID: 25128817 Free PMC article.
References
-
- Adams J. C., Zhang L. cDNA cloning of human muskelin and localization of the muskelin (MKLN1) gene to human chromosome 7q32 and mouse chromosome 6 B1/B2 by physical mapping and FISH. Cytogenet. Cell Genet. 1999;87:19–21. - PubMed
-
- Adams J. Characterization of a Drosophila melanogaster orthologue of muskelin. Gene. 2002;297:69–78. - PubMed
-
- Smith D. S., Niethammer M., Ayala R., Zhou Y., Gambello M. J., Wynshaw-Boris A., Tsai L. H. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat. Cell Biol. 2000;2:767–775. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

