Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer's disease
- PMID: 15084661
- PMCID: PMC6729359
- DOI: 10.1523/JNEUROSCI.5543-03.2004
Dimeric amyloid beta protein rapidly accumulates in lipid rafts followed by apolipoprotein E and phosphorylated tau accumulation in the Tg2576 mouse model of Alzheimer's disease
Abstract
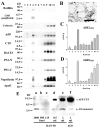
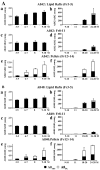
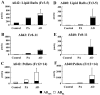
To investigate lipid rafts as a site where amyloid beta protein (Abeta) oligomers might accumulate and cause toxicity in Alzheimer's disease (AD), we analyzed Abeta in the Tg2576 transgenic mouse model of AD. Abeta was highly concentrated in lipid rafts, which comprise a small fraction of brain volume but contain 27% of brain Abeta42 and 24% of Abeta40 in young mice. In the Tg2576 model, memory impairment begins at 6 months before amyloid plaques are visible. Here we show that Abeta dimers appear in lipid rafts at 6 months and that raft Abeta, which is primarily dimeric, rapidly accumulates reaching levels >500x those in young mice by 24-28 months. A similar large accumulation of dimeric Abeta was observed in lipid rafts from AD brain. In contrast to extracellular amyloid fibrils, which are SDS-insoluble, virtually all Abeta in lipid rafts is SDS soluble. Coupled with recent studies showing that synthetic and naturally occurring Abeta oligomers can inhibit hippocampal long-term potentiation, the in vivo age-dependent accumulation of SDS-soluble Abeta dimers in lipid rafts at the time when memory impairment begins in Tg2576 mice provides strong evidence linking Abeta oligomers to memory impairment. After dimeric Abeta began to accumulate in lipid rafts of the Tg2576 brain, apolipoprotein E (ApoE) and then phosphorylated tau accumulated. A similar increase in ApoE and a large increase in phosphorylated tau was observed in lipid rafts from AD brain. These findings suggest that lipid rafts may be an important site for interaction between dimeric Abeta, ApoE, and tau.
Figures

References
-
- Avdulov NA, Chochina SV, Igbavboa U, Warden CS, Vassiliev AV, Wood WG (1997) Lipid binding to amyloid β-peptide aggregates: preferential binding of cholesterol as compared with phosphatidylcholine and fatty acids. J Neurochem 69: 1746–1752. - PubMed
-
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM (1997) Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet 17: 263–264. - PubMed
-
- Bickel PE, Scherer PE, Schnitzer JE, Oh P, Lisanti MP, Lodish HF (1997) Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem 272: 13793–13802. - PubMed
-
- Brown DA, London E (1998) Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol 14: 111–136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
