Arabidopsis AtCYP20-2 is a light-regulated cyclophilin-type peptidyl-prolyl cis-trans isomerase associated with the photosynthetic membranes
- PMID: 15084723
- PMCID: PMC419801
- DOI: 10.1104/pp.104.041186
Arabidopsis AtCYP20-2 is a light-regulated cyclophilin-type peptidyl-prolyl cis-trans isomerase associated with the photosynthetic membranes
Figures

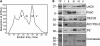
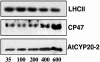
Comment in
-
Introducing immunophilins. From organ transplantation to plant biology.Plant Physiol. 2004 Apr;134(4):1241-3. doi: 10.1104/pp.103.900108. Plant Physiol. 2004. PMID: 15084722 Free PMC article. No abstract available.
References
-
- Anderson JM, Chow WS, Goodchild DJ (1988) Thylakoid membrane organisation in sun/shade acclimation. Aust J Plant Physiol 15: 11–26
-
- Bailey S, Walters RG, Jansson S, Horton P (2001) Acclimation of Arabidopsis thaliana to the light environment: the existence of separate low light and high light responses. Planta 213: 794–801 - PubMed
-
- Boekema EJ, van Roon H, Calkoen F, Bass R, Dekker JP (1999. a) Multiple types of association of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Biochemistry 38: 2233–2239 - PubMed
-
- Boekema EJ, van Roon H, Van Breemen JFL, Dekker JP (1999. b) Supramolecular organization of photosystem II and its light-harvesting antenna in partially solubilized photosystem II membranes. Eur J Biochem 266: 444–452 - PubMed
-
- Boekema EJ, Van Breemen JFL, Van Roon H, Dekker JP (2000) Arrangement of photosystem II supercomplexes in crystalline macrodomains within the thylakoid membrane of green plant chloroplasts. J Mol Biol 301: 1123–1133 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

