Phosphorylation of the amino terminus of maize sucrose synthase in relation to membrane association and enzyme activity
- PMID: 15084730
- PMCID: PMC419819
- DOI: 10.1104/pp.103.036780
Phosphorylation of the amino terminus of maize sucrose synthase in relation to membrane association and enzyme activity
Abstract
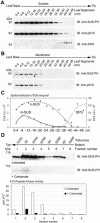
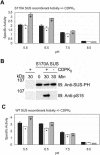
Sucrose synthase (SUS) is phosphorylated on a major, amino-terminal site located at Ser-15 (S15) in the maize (Zea mays) SUS1 protein. Site- and phospho-specific antibodies against a phosphorylated S15 (pS15) peptide allowed direct analysis of S15 phosphorylation in relation to membrane association. Immunoblots of the maize leaf elongation zone, divided into 4-cm segments, demonstrated that the abundance of soluble (s-SUS) and membrane (m-SUS) SUS protein showed distinct positional profiles. The content of m-SUS was maximal in the 4- to 8-cm segment where it represented 9% of total SUS and occurred as a peripheral membrane protein. In contrast, s-SUS was highest in the 12- to 16-cm segment. Relative to s-SUS, m-SUS was hypophosphorylated at S15 in the basal 4 cm but hyperphosphorylated in apical segments. Differing capabilities of the anti-pS15 and anti-S15 peptide antibodies to immunoprecipitate SUS suggested that phosphorylation of S15, or exposure of unphosphorylated SUS to slightly acidic pH, altered the structure of the amino terminus. These structural changes were generally coincident with the increased sucrose cleavage activity that occurs at pH values below 7.5. In vitro S15 phosphorylation of the S170A SUS protein by a maize calcium-dependent protein kinase (CDPK) significantly increased sucrose cleavage activity at low pH. Collectively, the results suggest that (1) SUS membrane binding is controlled in vivo; (2) relative pS15 content of m-SUS depends on the developmental state of the organ; and (3) phosphorylation of S15 affects amino-terminal conformation in a way that may stimulate the catalytic activity of SUS and influence membrane association.
Figures



References
-
- Anguenot R, Yelle S, Nguyen-Quoc B (1999) Purification of tomato sucrose synthase phosphorylated isoforms by Fe(III)-immobilized metal affinity chromatography. Arch Biochem Biophys 365: 163–169 - PubMed
-
- Azama K, Abe S, Sugimoto H, Davies E (2003) Lysine-containing proteins in maize endosperm: a major contribution from cytoskeleton-associated carbohydrate-metabolizing enzymes. Planta 217: 628–638 - PubMed
-
- Bell-Parikh LC, Eipper BA, Mains RE (2001) Response of an integral granule membrane protein to changes in pH. J Biol Chem 276: 29854–29863 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

