Lactosylceramide recruits PKCalpha/epsilon and phospholipase A2 to stimulate PECAM-1 expression in human monocytes and adhesion to endothelial cells
- PMID: 15084746
- PMCID: PMC404072
- DOI: 10.1073/pnas.0308684101
Lactosylceramide recruits PKCalpha/epsilon and phospholipase A2 to stimulate PECAM-1 expression in human monocytes and adhesion to endothelial cells
Abstract
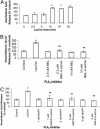
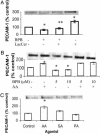
Despite the importance of platelet/endothelial cell adhesion molecule-1 (PECAM-1, CD31) in the adhesion and diapedesis of monocytes/lymphocytes, little is known about the mechanisms by which it is regulated. We explored the role of a glycosphingolipid, lactosylceramide (LacCer), in modulating PECAM-1 expression and cell adhesion in human monocytes. We observed that LacCer specifically exerted a time-dependent increase in PECAM-1 expression in U-937 cells. Maximal increase in PECAM-1 protein occurred after incubation with LacCer for 60 min. LacCer activated PKCalpha and -epsilon by translocating them from cytosol to membrane. This was accompanied by the activation of phospholipase A(2) (PLA(2)) and the increase of cell adhesion, which were abrogated by chelerythrine chloride, 2-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-3-(1H-indol-3-yl)-maleimide and 12-(2-cyanoethyl)-6,7,12,13-tetrahydro-13-methyl-5-oxo-5H-indolo(2,3-a)pyrrolo(3,4-c)-carbazole (GO 6976) (PKC inhibitors). Similarly, bromoenol lactone (a Ca(2+)-independent PLA(2) inhibitor) and methyl arachidonyl fluorophosphonate (an inhibitor of cytosolic PLA(2) and Ca(2+)-independent PLA(2)) inhibited LacCer-induced PLA(2) activity. Bromophenacyl bromide (a PLA(2) inhibitor) abrogated LacCer-induced PECAM-1 expression, and this was bypassed by arachidonic acid. Furthermore, the arachidonate-induced up-regulation of PECAM-1 was abrogated by indomethacin [a cyclooxygenase (COX)-1 and -2 inhibitor] or N-[2-(cyclohexyloxy)-4-nitrophenyl]-methanesulfonamide (a COX-2 inhibitor) but not nordihydroguaiaretic acid (a lipoxygenase inhibitor). In sum, PKCalpha/epsilon are the primary targets for the activation of LacCer. Downstream activation of intracellular Ca(2+)-independent PLA(2) and/or cytosolic PLA(2) results in the production of arachidonic acid, which in turn serves as a precursor for prostaglandins that subsequently stimulate PECAM-1 expression and cell adhesion. These findings may be relevant in explaining the role of LacCer in the regulation of PECAM-1 and related pathophysiology.
Figures




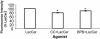
References
-
- Minehata, K., Mukouyama, Y. S., Sekiguchi, T., Hara, T. & Miyajima, A. (2002) Blood 99, 2360-2368. - PubMed
-
- Serebruany, V. L., Murugesan, S. R., Pothula, A., Atar, D., Lowry, D. R., O'Connor, C. M. & Gurbel, P. A. (1999) Eur. J. Heart Fail. 1, 243-249. - PubMed
-
- Serebruany, V. L., Murugesan, S. R., Pothula, A., Semaan, H. & Gurbel, P. A. (1999) Cardiology 91, 50-55. - PubMed
-
- Blann, A. D., Devine, C., Amiral, J. & McCollum, C. N. (1998) Blood Coagul. Fibrinolysis 9, 479-484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

