Doxycycline-a role in ocular surface repair
- PMID: 15090411
- PMCID: PMC1772144
- DOI: 10.1136/bjo.2003.025551
Doxycycline-a role in ocular surface repair
Abstract
Background/aims: Doxycycline is a broad spectrum antibiotic that chelates metal ions and is frequently used as part of the treatment of ocular surface diseases. Its therapeutic value has been ascribed to an ability to inhibit matrix metalloproteinase (MMP) activity and both MMP and IL-1 synthesis. The aim of this study was to evaluate the role of doxycycline as an inhibitor of corneal MMPs and assess its contribution to ocular surface repair mechanisms.
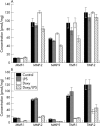
Methods: Corneal epithelial cell and keratocyte cultures were grown to confluence and incubated with IL-1alpha, LPS, doxycycline, or doxycycline and LPS in serum free medium for 4 days. The cells were either harvested and assayed for caspase-3 activity or stained with either AE5 or antivimentin antibodies. Media samples were concentrated and assayed for MMP activity by zymography or using a fluorigenic substrate. ELISA was used to quantify IL-1alpha, MMPs -1,-2,-3,-9, and TIMPs -1 and -2.
Results: IL-1alpha and LPS had no effect on MMP/TIMP production by cultured corneal epithelial cells and keratocytes. Corneal MMP-2 inhibition by doxycycline was partially [Ca(2+)] dependent but irreversible. At the minimum inhibitory concentration, 100 micro m, doxycycline had no apparent effect on MMP and TIMP production, but ultimately caused the death of keratocytes and some of the epithelial cells that detached from their basement membrane. Caspase-3 activity was not detected in dead or dying keratocytes. The mechanism of cell death in cultured corneal epithelial cells was not caspase-3 related apoptosis as the activity of this enzyme, normally detectable, was lost. The epithelial cells that survived doxycycline treatment did not bind antivimentin antibody and compared with controls, reacted less with the AE5 antibody. They were probably transient amplifying cells.
Conclusions: Doxycycline irreversibly inhibits corneal MMP-2 activity by chelating the metal ions that are catalytically and structurally essential. Corneal MMP/TIMP production in vitro is not modulated by IL-1alpha, LPS, or doxycycline. The therapeutic value of doxycycline may depend upon its effective concentration at the ocular surface and probably relates to its chelating properties.
Figures





References
-
- Carlini G. Bacteriological and clinical investigations of the activity of a new antibiotic, doxycycline. In: Proceedings of the 5th International Congress of Chemotherapy, Vienna. 1967:457–72.
-
- Smith GN, Mickler EA, Hasty KA, et al. Specificity of inhibition of matrix metalloproteinase activity by doxycycline: Relationship to structure of the enzyme. Arthritis Rheum 1999;42:1140–6. - PubMed
-
- Ryan ME, Usman A, Ramamurthy NS, et al. Excessive matrix metalloproteinase activity in diabetes: Inhibition by tetracycline analogues with zinc reactivity. Curr Med Chem 2001;8:305–16. - PubMed
-
- Nordstrom D, Lindy O, Lauhio A, et al. Anti-collagenolytic mechanism of action of action of doxycycline treatment in rheumatoid arthritis. Rheumatol Int 1998;17:175–80. - PubMed
-
- Golub LM, Sorsa T, Lee HM, et al. Doxycycline inhibits neutrophil (PMN)-type matrix metalloproteinases in human adult periodontitis. J Clin Peridontol 1995;22:100–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
