The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator
- PMID: 15090506
- PMCID: PMC387786
- DOI: 10.1128/JB.186.9.2655-2663.2004
The membrane fluidity sensor DesK of Bacillus subtilis controls the signal decay of its cognate response regulator
Abstract
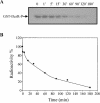
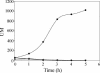
The Bacillus subtilis DesK/DesR two-component system regulates the expression of the des gene coding for the Delta5 acyl lipid desaturase. It is believed that a decrease in membrane lipid fluidity activates the DesK/DesR signal transduction cascade, which results in synthesis of the Delta5 acyl lipid desaturase and desaturation of membrane phospholipids. These newly synthesized unsaturated fatty acids then act as negative signals of des transcription, thus generating a regulatory metabolic loop that optimizes membrane fluidity. We previously suggested that DesK is a bifunctional enzyme with both kinase and phosphatase activities that could assume different signaling states in response to changes in the fluidity of membrane lipids. However, no direct experimental evidence supported this proposed model. In this study, we show that the C-terminal fragment of the DesK protein (DesKC) indeed acts as an autokinase. Addition of the response regulator DesR to phosphorylated DesKC resulted in rapid transfer of the phosphoryl group to DesR. Further, phosphorylated DesR can be dephosphorylated in the presence of DesKC, thus demonstrating that the sensor kinase has the ability to covalently modify DesR through both kinase and phosphatase activities. We also present evidence that DesKC might be locked in a kinase-dominant state in vivo and that its activities are not affected either in vivo or in vitro by unsaturated fatty acids. These findings provide the first direct evidence that the transmembrane segments of DesK are essential to sense changes in membrane fluidity and for regulating the ratio of kinase to phosphatase activities of the cytoplasmic C-terminal domain.
Figures




References
-
- Castelli, M. E., V. E. Garcia, and F. C. Soncini. 2000. The phosphatase activity is the target for Mg2+ regulation of the sensor protein PhoQ in Salmonella. J. Biol. Chem. 275:22948-22954. - PubMed
-
- Cybulski, L. E., D. Albanesi, M. C. Mansilla, S. Altabe, P. S. Aguilar, and D. de Mendoza. 2002. Mechanism of membrane fluidity optimization: isothermal control of the Bacillus subtilis acyl-lipid desaturase. Mol. Microbiol. 45:1379-1388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

