Nerve injury reduces responses of hypoglossal motoneurones to baseline and chemoreceptor-modulated inspiratory drive in the adult rat
- PMID: 15090609
- PMCID: PMC1665144
- DOI: 10.1113/jphysiol.2003.059972
Nerve injury reduces responses of hypoglossal motoneurones to baseline and chemoreceptor-modulated inspiratory drive in the adult rat
Abstract
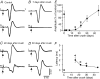
The effects of peripheral nerve lesions on the membrane and synaptic properties of motoneurones have been extensively studied. However, minimal information exists about how these alterations finally influence discharge activity and motor output under physiological afferent drive. The aim of this work was to evaluate the effect of hypoglossal (XIIth) nerve crushing on hypoglossal motoneurone (HMN) discharge in response to the basal inspiratory afferent drive and its chemosensory modulation by CO(2). The evolution of the lesion was assessed by recording the compound muscle action potential evoked by XIIth nerve stimulation, which was lost on crushing and then recovered gradually to control values from the second to fourth weeks post-lesion. Basal inspiratory activities recorded 7 days post-injury in the nerve proximal to the lesion site, and in the nucleus, were reduced by 51.6% and 35.8%, respectively. Single unit antidromic latencies were lengthened by lesion, and unusually high stimulation intensities were frequently required to elicit antidromic spikes. Likewise, inspiratory modulation of unitary discharge under conditions in which chemoreceptor drive was varied by altering end-tidal CO(2) was reduced by more than 60%. Although the general recruitment scheme was preserved after XIIth nerve lesion, we noticed an increased proportion of low-threshold units and a reduced recruitment gain across the physiological range. Immunohistochemical staining of synaptophysin in the hypoglossal nuclei revealed significant reductions of this synaptic marker after nerve injury. Morphological and functional alterations recovered with muscle re-innervation. Thus, we report here that nerve lesion induced changes in the basal activity and discharge modulation of HMNs, concurrent with the loss of afferent inputs. Nevertheless, we suggest that an increase in membrane excitability, reported by others, and in the proportion of low-threshold units, could serve to preserve minimal electrical activity, prevent degeneration and favour axonal regeneration.
Figures
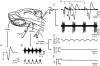








Similar articles
-
Arterial chemoreceptor input to respiratory hypoglossal motoneurons.J Appl Physiol (1985). 1990 Aug;69(2):700-9. doi: 10.1152/jappl.1990.69.2.700. J Appl Physiol (1985). 1990. PMID: 2121703
-
Difference between hypoglossal and phrenic activities during lung inflation and swallowing in the rat.J Physiol. 2002 Oct 1;544(Pt 1):183-93. doi: 10.1113/jphysiol.2002.022566. J Physiol. 2002. PMID: 12356891 Free PMC article.
-
Estimation of postsynaptic potentials in rat hypoglossal motoneurones: insights for human work.J Physiol. 2003 Sep 1;551(Pt 2):419-31. doi: 10.1113/jphysiol.2003.044982. Epub 2003 Jul 18. J Physiol. 2003. PMID: 12872008 Free PMC article.
-
Drive to the human respiratory muscles.Respir Physiol Neurobiol. 2007 Nov 15;159(2):115-26. doi: 10.1016/j.resp.2007.06.006. Epub 2007 Jun 17. Respir Physiol Neurobiol. 2007. PMID: 17660051 Review.
-
Optimization character of inspiratory neural drive.J Appl Physiol (1985). 1992 May;72(5):2005-17. doi: 10.1152/jappl.1992.72.5.2005. J Appl Physiol (1985). 1992. PMID: 1601812 Review.
Cited by
-
Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis.J Neurosci. 2008 Oct 22;28(43):10864-74. doi: 10.1523/JNEUROSCI.1340-08.2008. J Neurosci. 2008. PMID: 18945894 Free PMC article.
-
Reduction in the motoneuron inhibitory/excitatory synaptic ratio in an early-symptomatic mouse model of amyotrophic lateral sclerosis.Brain Pathol. 2011 Jan;21(1):1-15. doi: 10.1111/j.1750-3639.2010.00417.x. Brain Pathol. 2011. PMID: 20653686 Free PMC article.
-
Nitric oxide induces pathological synapse loss by a protein kinase G-, Rho kinase-dependent mechanism preceded by myosin light chain phosphorylation.J Neurosci. 2010 Jan 20;30(3):973-84. doi: 10.1523/JNEUROSCI.3911-09.2010. J Neurosci. 2010. PMID: 20089906 Free PMC article.
-
New insight into the antifibrotic effects of praziquantel on mice in infection with Schistosoma japonicum.PLoS One. 2011;6(5):e20247. doi: 10.1371/journal.pone.0020247. Epub 2011 May 24. PLoS One. 2011. PMID: 21629648 Free PMC article.
-
Neuroprotective effect of testosterone treatment on motoneuron recruitment following the death of nearby motoneurons.Dev Neurobiol. 2009 Oct;69(12):825-35. doi: 10.1002/dneu.20743. Dev Neurobiol. 2009. PMID: 19658088 Free PMC article.
References
-
- Brännström T, Kellerth JO. Changes in synaptology of adult cat spinal α-motoneurons after axotomy. Exp Brain Res. 1998;118:1–13. - PubMed
-
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. - PubMed
-
- Cohen MI. Discharge patterns of brain-stem respiratory neurons in relation to carbon dioxide tension. J Neurophysiol. 1968;31:142–165. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

