Decrease in arterial pressure induced by adrenomedullin in the hypothalamic paraventricular nucleus is mediated by nitric oxide and GABA
- PMID: 15093693
- PMCID: PMC4820401
- DOI: 10.1016/j.regpep.2003.12.018
Decrease in arterial pressure induced by adrenomedullin in the hypothalamic paraventricular nucleus is mediated by nitric oxide and GABA
Abstract
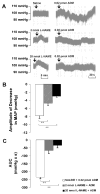
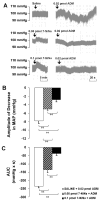
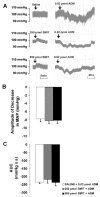
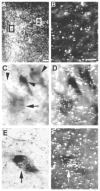
We tested the hypothesis that the decrease in arterial pressure induced by adrenomedullin (ADM) in the hypothalamic paraventricular nucleus (PVN) is mediated by nitric oxide (NO) and/or GABA. Unilateral microinjections of ADM into the PVN of anesthetized rats caused a significant decrease in mean arterial pressure (MAP). The ADM-induced decrease in MAP was significantly attenuated by pretreatment with N(psi)-nitro-L-arginine methyl ester (L-NAME, a non-selective NOS inhibitor), 7-nitroindazole sodium salt (7-NiNa, a selective neuronal NOS inhibitor), N5-(1-Iminoethyl)-L-ornithine (L-NIO, a selective endothelial NOS inhibitor) or bicuculline methiodide, but pretreatment with S-methylisothiourea (SMIT, a selective inducible NOS inhibitor) had no effect on this ADM-induced effect. In addition, coronal sections of rat brains were processed for combined NADPH-diaphorase (a marker of neuronal NOS-containing neurons) histochemistry and in situ hybridization for the receptor-activity-modifying protein 2 (a specific ADM receptor component). Double-labeled neurons were found in both parvocellular and magnocellular subdivisions of the PVN, confirming that NO-producing neurons in the PVN are capable of mediating ADM's effects. Thus, our data provide evidence that the ADM-induced decrease in MAP in the PVN is mediated by NO from neuronal and endothelial NOS, and by GABA.
Figures



Similar articles
-
Adrenomedullin acts in the lateral parabrachial nucleus to increase arterial blood pressure through mechanisms mediated by glutamate and nitric oxide.Am J Physiol Regul Integr Comp Physiol. 2008 Jul;295(1):R38-44. doi: 10.1152/ajpregu.00172.2008. Epub 2008 May 21. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18495835
-
Adrenomedullin in paraventricular nucleus attenuates adipose afferent reflex and sympathoexcitation via receptors mediated nitric oxide-gamma-aminobutyric acid A type receptor pathway in rats with obesity-related hypertension.J Hypertens. 2023 Feb 1;41(2):233-245. doi: 10.1097/HJH.0000000000003301. J Hypertens. 2023. PMID: 36583351
-
Estrogen in the paraventricular nucleus attenuates L-glutamate-induced increases in mean arterial pressure through estrogen receptor beta and NO.Hypertension. 2006 Dec;48(6):1130-6. doi: 10.1161/01.HYP.0000248754.67128.ff. Epub 2006 Oct 30. Hypertension. 2006. PMID: 17075034
-
Nitric oxide synthase inhibitors. Preclinical studies of potential use for treatment of opioid withdrawal.Neuropsychopharmacology. 1995 Dec;13(4):315-22. doi: 10.1016/0893-133X(95)00138-4. Neuropsychopharmacology. 1995. PMID: 8747756 Review.
-
The hypothalamus and hypertension.Physiol Rev. 2001 Oct;81(4):1599-658. doi: 10.1152/physrev.2001.81.4.1599. Physiol Rev. 2001. PMID: 11581498 Review.
Cited by
-
Is endothelial-nitric-oxide-synthase-derived nitric oxide involved in cardiac hypoxia/reoxygenation-related damage?J Biosci. 2011 Mar;36(1):69-78. doi: 10.1007/s12038-011-9006-4. J Biosci. 2011. PMID: 21451249
-
Adrenomedullin and Adrenomedullin-Targeted Therapy As Treatment Strategies Relevant for Sepsis.Front Immunol. 2018 Feb 19;9:292. doi: 10.3389/fimmu.2018.00292. eCollection 2018. Front Immunol. 2018. PMID: 29520277 Free PMC article. Review.
-
Adrenomedullin in the rostral ventrolateral medulla inhibits baroreflex control of heart rate: a role for protein kinase A.Br J Pharmacol. 2006 May;148(1):70-7. doi: 10.1038/sj.bjp.0706698. Br J Pharmacol. 2006. PMID: 16501581 Free PMC article.
-
Adrenomedullin in the rostral ventrolateral medulla increases arterial pressure and heart rate: roles of glutamate and nitric oxide.Am J Physiol Regul Integr Comp Physiol. 2004 Oct;287(4):R729-34. doi: 10.1152/ajpregu.00188.2004. Epub 2004 Jun 3. Am J Physiol Regul Integr Comp Physiol. 2004. PMID: 15178541 Free PMC article.
-
Activation of estrogen receptor beta-dependent nitric oxide signaling mediates the hypotensive effects of estrogen in the rostral ventrolateral medulla of anesthetized rats.J Biomed Sci. 2009 Jul 7;16(1):60. doi: 10.1186/1423-0127-16-60. J Biomed Sci. 2009. PMID: 19583861 Free PMC article.
References
-
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matsuo H, et al. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochem Biophys Res Commun. 1993;192:553–60. - PubMed
-
- Christopoulos G, Perry KJ, Morfis M, Tilakaratne N, Gao Y, Fraser NJ, et al. Multiple amylin receptors arise from receptor activity-modifying protein interaction with the calcitonin receptor gene product. Mol Pharmacol. 1999;56:235–42. - PubMed
-
- Flahaut M, Rossier BC, Firsov D. Respective roles of calcitonin receptor-like receptor (crlr) and receptor activity-modifying proteins (ramp) in cell surface expression of crlr/ramp heterodimeric receptors. J Biol Chem. 2002;277:14731–7. - PubMed
-
- Fraser NJ, Wise A, Brown J, McLatchie LM, Main MJ, Foord SM. The amino terminus of receptor activity modifying proteins is a critical determinant of glycosylation state and ligand binding of calcitonin receptor-like receptor. Mol Pharmacol. 1999;55:1054–9. - PubMed
-
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. Ramps regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

