Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation
- PMID: 15096539
- PMCID: PMC1361263
- DOI: 10.1084/jem.20031560
Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation
Abstract
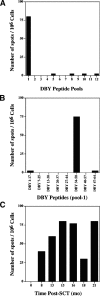
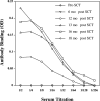
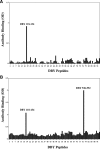
We examined the immune response to DBY, a model H-Y minor histocompatibility antigen (mHA) in a male patient with chronic graft-versus-host disease (GVHD) after allogeneic hematopoietic stem cell transplant from a human histocompatibility leukocyte antigen (HLA)-identical female sibling. Patient peripheral blood mononuclear cells were screened for reactivity against a panel of 93 peptides representing the entire amino acid sequence of DBY. This epitope screen revealed a high frequency CD4(+) T cell response to a single DBY peptide that persisted from 8 to 21 mo after transplant. A CD4(+) T cell clone displaying the same reactivity was established from posttransplant patient cells and used to characterize the T cell epitope as a 19-mer peptide starting at position 30 in the DBY sequence and restricted by HLA-DRB1*1501. Remarkably, the corresponding X homologue peptide was also recognized by donor T cells. Moreover, the T cell clone responded equally to mature HLA-DRB1*1501 male and female dendritic cells, indicating that both DBY and DBX peptides were endogenously processed. After transplant, the patient also developed antibodies that were specific for recombinant DBY protein and did not react with DBX. This antibody response was mapped to two DBY peptides beginning at positions 118 and 536. Corresponding DBX peptides were not recognized. These studies provide the first demonstration of a coordinated B and T cell immune response to an H-Y antigen after allogeneic transplant. The specificity for recipient male cells was mediated by the B cell response and not by donor T cells. This dual DBX/DBY antigen is the first mHA to be identified in the context of chronic GVHD.
Figures





Similar articles
-
Combined CD4 T-cell and antibody response to human minor histocompatibility antigen DBY after allogeneic stem-cell transplantation.Transplantation. 2011 Aug 15;92(3):359-65. doi: 10.1097/TP.0b013e3182244cc3. Transplantation. 2011. PMID: 21709606 Free PMC article.
-
Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors.Blood. 2004 Jan 1;103(1):353-9. doi: 10.1182/blood-2003-03-0984. Epub 2003 Sep 25. Blood. 2004. PMID: 14512314 Free PMC article.
-
The DBY gene codes for an HLA-DQ5-restricted human male-specific minor histocompatibility antigen involved in graft-versus-host disease.Blood. 2002 Apr 15;99(8):3027-32. doi: 10.1182/blood.v99.8.3027. Blood. 2002. PMID: 11929796
-
Negative depletion of α/β+ T cells and of CD19+ B lymphocytes: a novel frontier to optimize the effect of innate immunity in HLA-mismatched hematopoietic stem cell transplantation.Immunol Lett. 2013 Sep-Oct;155(1-2):21-3. doi: 10.1016/j.imlet.2013.09.027. Epub 2013 Sep 30. Immunol Lett. 2013. PMID: 24091162 Review.
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
Cited by
-
B-cell targeting in chronic graft-versus-host disease.Blood. 2018 Mar 29;131(13):1399-1405. doi: 10.1182/blood-2017-11-784017. Epub 2018 Feb 1. Blood. 2018. PMID: 29437591 Free PMC article. Review.
-
Beyond citrullination: other post-translational protein modifications in rheumatoid arthritis.Nat Rev Rheumatol. 2017 Jun;13(6):331-339. doi: 10.1038/nrrheum.2017.15. Epub 2017 Mar 9. Nat Rev Rheumatol. 2017. PMID: 28275265 Review.
-
Donor-recipient mismatch for common gene deletion polymorphisms in graft-versus-host disease.Nat Genet. 2009 Dec;41(12):1341-4. doi: 10.1038/ng.490. Nat Genet. 2009. PMID: 19935662 Free PMC article.
-
Combined CD4 T-cell and antibody response to human minor histocompatibility antigen DBY after allogeneic stem-cell transplantation.Transplantation. 2011 Aug 15;92(3):359-65. doi: 10.1097/TP.0b013e3182244cc3. Transplantation. 2011. PMID: 21709606 Free PMC article.
-
A uniform genomic minor histocompatibility antigen typing methodology and database designed to facilitate clinical applications.PLoS One. 2006 Dec 20;1(1):e42. doi: 10.1371/journal.pone.0000042. PLoS One. 2006. PMID: 17183671 Free PMC article.
References
-
- Simpson, E., D. Scott, E. James, G. Lombardi, K. Cwynarski, F. Dazzi, M. Millrain, and P.J. Dyson. 2002. Minor H antigens: genes and peptides. Transpl. Immunol. 10:115–123. - PubMed
-
- Falkenburg, J.H., W.A. Marijt, M.H. Heemskerk, and R. Willemze. 2002. Minor histocompatibility antigens as targets of graft-versus-leukemia reactions. Curr. Opin. Hematol. 9:497–502. - PubMed
-
- Riddell, S.R., M. Murata, S. Bryant, and E.H. Warren. 2002. Minor histocompatibility antigens–targets of graft versus leukemia responses. Int. J. Hematol. 76:155–161. - PubMed
-
- den Haan, J.M., N.E. Sherman, E. Blokland, E. Huczko, F. Koning, J.W. Drijfhout, J. Skipper, J. Shabanowitz, D.F. Hunt, V.H. Engelhard, et al. 1995. Identification of a graft versus host disease-associated human minor histocompatibility antigen. Science. 268:1476–1480. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

