PML-RARA-RXR oligomers mediate retinoid and rexinoid/cAMP cross-talk in acute promyelocytic leukemia cell differentiation
- PMID: 15096541
- PMCID: PMC2211888
- DOI: 10.1084/jem.20032226
PML-RARA-RXR oligomers mediate retinoid and rexinoid/cAMP cross-talk in acute promyelocytic leukemia cell differentiation
Abstract
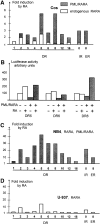
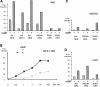
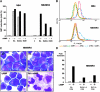
PML-RARA was proposed to initiate acute promyelocytic leukemia (APL) through PML-RARA homodimer-triggered repression. Here, we examined the nature of the PML-RARA protein complex and of its DNA targets in APL cells. Using a selection/amplification approach, we demonstrate that PML-RARA targets consist of two AGGTCA elements in an astonishing variety of orientations and spacings, pointing to highly relaxed structural constrains for DNA binding and identifying a major gain of function of this oncogene. PML-RARA-specific response elements were identified, which all conveyed a major transcriptional response to RA only in APL cells. In these cells, we demonstrate that PML-RARA oligomers are complexed to RXR. Directly probing PML-RARA function in APL cells, we found that the differentiation enhancer cyclic AMP (cAMP) boosted transcriptional activation by RA. cAMP also reversed the normal silencing (subordination) of the transactivating function of RXR when bound to RARA or PML-RARA, demonstrating that the alternate rexinoid/cAMP-triggered APL differentiation pathway also activates PML-RARA targets. Finally, cAMP restored both RA-triggered differentiation and PML-RARA transcriptional activation in mutant RA-resistant APL cells. Collectively, our findings directly demonstrate that APL cell differentiation parallels transcriptional activation through PML-RARA-RXR oligomers and that those are functionally targeted by cAMP, identifying this agent as another oncogene-targeted therapy.
Figures

Similar articles
-
Uncoupling RARA transcriptional activation and degradation clarifies the bases for APL response to therapies.J Exp Med. 2013 Apr 8;210(4):647-53. doi: 10.1084/jem.20122337. Epub 2013 Mar 18. J Exp Med. 2013. PMID: 23509325 Free PMC article.
-
Novel treatment of acute promyelocytic leukemia: As₂O₃, retinoic acid and retinoid pharmacology.Curr Pharm Biotechnol. 2013;14(9):849-58. doi: 10.2174/1389201015666140113095812. Curr Pharm Biotechnol. 2013. PMID: 24433507 Review.
-
cAMP signalling is decisive for recovery of nuclear bodies (PODs) during maturation of RA-resistant t(15;17) promyelocytic leukemia NB4 cells expressing PML-RAR alpha.Oncogene. 1996 Jun 6;12(11):2451-9. Oncogene. 1996. PMID: 8649787
-
Differential changes of retinoid-X-receptor (RXR alpha) and its RAR alpha and PML-RAR alpha partners induced by retinoic acid and cAMP distinguish maturation sensitive and resistant t(15;17) promyelocytic leukemia NB4 cells.Oncogene. 1996 Jun 6;12(11):2443-50. Oncogene. 1996. PMID: 8649786
-
Therapy-induced PML/RARA proteolysis and acute promyelocytic leukemia cure.Clin Cancer Res. 2009 Oct 15;15(20):6321-6. doi: 10.1158/1078-0432.CCR-09-0209. Epub 2009 Oct 6. Clin Cancer Res. 2009. PMID: 19808868 Review.
Cited by
-
Effects of PPARγ Ligands on Leukemia.PPAR Res. 2012;2012:483656. doi: 10.1155/2012/483656. Epub 2012 May 21. PPAR Res. 2012. PMID: 22685453 Free PMC article.
-
Perspectives of differentiation therapies of acute myeloid leukemia: the search for the molecular basis of patients' variable responses to 1,25-dihydroxyvitamin d and vitamin d analogs.Front Oncol. 2014 May 27;4:125. doi: 10.3389/fonc.2014.00125. eCollection 2014. Front Oncol. 2014. PMID: 24904835 Free PMC article. Review.
-
Dominant-negative mechanism of leukemogenic PAX5 fusions.Oncogene. 2012 Feb 23;31(8):966-77. doi: 10.1038/onc.2011.291. Epub 2011 Jul 18. Oncogene. 2012. PMID: 21765475 Free PMC article.
-
Acute promyelocytic leukaemia: novel insights into the mechanisms of cure.Nat Rev Cancer. 2010 Nov;10(11):775-83. doi: 10.1038/nrc2943. Epub 2010 Oct 22. Nat Rev Cancer. 2010. PMID: 20966922 Review.
-
Interaction with RXR is necessary for NPM-RAR-induced myeloid differentiation blockade.Leuk Res. 2013 Dec;37(12):1704-10. doi: 10.1016/j.leukres.2013.09.024. Epub 2013 Sep 29. Leuk Res. 2013. PMID: 24183235 Free PMC article.
References
-
- Castelein, H., A. Janssen, P.E. Declercq, and M. Baes. 1996. Sequence requirements for high affinity retinoid X receptor-alpha homodimer binding. Mol. Cell. Endocrinol. 119:11–20. - PubMed
-
- Forman, B.M., K. Umesono, J. Chen, and R.M. Evans. 1995. Unique response pathways are established by allosteric interactions among nuclear hormone receptors. Cell. 81:541–550. - PubMed
-
- Germain, P., J. Iyer, C. Zechel, and H. Gronemeyer. 2002. Co-regulator recruitment and the mechanism of retinoic acid receptor synergy. Nature. 415:187–192. - PubMed
-
- Minucci, S., C. Nervi, F. Lo Coco, and P.G. Pelicci. 2001. Histone deacetylases: a common molecular target for differentiation treatment of acute myeloid leukemias? Oncogene. 20:3110–3115. - PubMed
-
- Jansen, J.H., A. Mahfoudi, S. Rambaud, C. Lavau, W. Wahli, and A. Dejean. 1995. Multimeric complexes of the PML-retinoic acid receptor alpha fusion protein in acute promyelocytic leukemia cells and interference with retinoid and peroxisome-proliferator signaling pathways. Proc. Natl. Acad. Sci. USA. 92:7401–7405. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

