Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver
- PMID: 15096593
- PMCID: PMC404058
- DOI: 10.1073/pnas.0401627101
Stearoyl-CoA desaturase 1 deficiency increases fatty acid oxidation by activating AMP-activated protein kinase in liver
Abstract
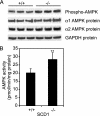
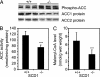
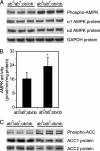
Stearoyl-CoA desaturase (SCD) catalyzes the rate-limiting step in the biosynthesis of monounsaturated fatty acids. Mice with a targeted disruption of the SCD1 isoform have reduced body adiposity, increased energy expenditure, and up-regulated expression of several genes encoding enzymes of fatty acid beta-oxidation in liver. The mechanisms by which SCD deficiency leads to these metabolic changes are presently unknown. Here we show that the phosphorylation and activity of AMP-activated protein kinase (AMPK), a metabolic sensor that regulates lipid metabolism during increased energy expenditure is significantly increased (approximately 40%, P < 0.01) in liver of SCD1 knockout mice (SCD1-/-). In parallel with the activation of AMPK, the phosphorylation of acetyl-CoA carboxylase at Ser-79 was increased and enzymatic activity was decreased (approximately 35%, P < 0.001), resulting in decreased intracellular levels of malonyl-CoA (approximately 47%, P < 0.001). An SCD1 mutation also increased AMPK phosphorylation and activity and increased acetyl-CoA carboxylase phosphorylation in leptin-deficient ob/ob mice. Lower malonyl-CoA concentrations are known to derepress carnitine palmitoyltransferase 1 (CPT1). In SCD1-/- mice, CPT1 and CPT2 activities were significantly increased (in both cases approximately 60%, P < 0.001) thereby stimulating the oxidation of mitochondrial palmitoyl-CoA. Our results identify AMPK as a mediator of increased fatty acid oxidation in liver of SCD1-deficient mice.
Figures



References
-
- Enoch, H. G., Catala, A. & Strittmatter, P. (1976) J. Biol. Chem. 251, 5095-5103. - PubMed
-
- Ntambi, J. M. (1999) J. Lipid Res. 40, 1549-1558. - PubMed
-
- Miyazaki, M., Man, W. C. & Ntambi, J. M. (2001) J. Nutr. 131, 2260-2268. - PubMed
-
- Miyazaki, M., Kim, Y. C., Gray-Keller, M. P., Attie, A. D. & Ntambi, J. M. (2000) J. Biol. Chem. 275, 30132-30138. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

