Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis
- PMID: 15096601
- PMCID: PMC404052
- DOI: 10.1073/pnas.0401855101
Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis
Abstract
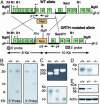
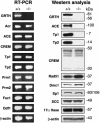
Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25), a member of the DEAD-box protein family, is a testis-specific gonadotropin-regulated RNA helicase that is present in Leydig cells and germ cells (meiotic spermatocytes and spermatids). In this study, we observed that GRTH is present in the nucleus, cytoplasm and chromatoid body of germ cells, and is an integral component of messenger ribonuclear protein particles. Male mice with a null mutation in the GRTH gene displayed normal gonadotropin and androgen profiles. However, they were sterile, with azoospermia caused by a complete arrest of spermiogenesis at step 8 of round spermatids and failure to elongate. Round spermatids of the null mice showed marked diminution in the size of chromatoid bodies. The transcription of relevant messages was not altered, but their translation was abrogated in a selective manner. Protein expression of transition proteins 1 and 2 and angiotensin-converting enzyme was completely absent, whereas that of the transcriptional activator cAMP responsive element modulator was intact. These findings indicate that GRTH participates in translational-associated events during germ cell development. Although significant apoptosis was present at the metaphase of meiosis in the GRTH-null mice, spermatogenesis proceeded to step 8 of spermiogenesis when complete arrest occurred. This progression may relate to compensatory gene function(s) and/or the observed up-regulation of DNA repair proteins Rad51 and Dmc1. This study (i) demonstrates that GRTH is essential for completion of spermatogenesis, (ii) provides insights into intrinsic requirements for spermiogenesis, and (iii) establishes a model for studies of male infertility and contraception.
Figures



References
-
- Steger, K. (2001) Anat. Embryol. (Berlin) 203, 323-334. - PubMed
-
- Eddy, E. M. (2002) Recent Prog. Horm. Res. 57, 103-128. - PubMed
-
- Schmid, S. R. & Linder, P. (1992) Mol. Microbiol. 6, 283-291. - PubMed
-
- de la Cruz, J., Kressler, D. & Linder, P. (1999) Trends Biochem. Sci. 24, 192-198. - PubMed
-
- Silverman, E., Edwalds-Gilbert, G. & Lin, R. J. (2003) Gene 312, 1-16. - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

