A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl(-) channel
- PMID: 15096609
- PMCID: PMC404120
- DOI: 10.1073/pnas.0401604101
A role of reactive oxygen species in apoptotic activation of volume-sensitive Cl(-) channel
Abstract
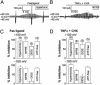
Apoptotic volume decrease is a pivotal event triggering a cell to undergo apoptosis and is induced by ionic effluxes resulting mainly from increased K(+) and Cl(-) conductances. Here, we demonstrate that in human epithelia HeLa cells both mitochondrion- and death receptor-mediated apoptosis inducers [staurosporine and Fas ligand or tumor necrosis factor (TNF)-alpha] rapidly activate Cl(-) currents that show properties phenotypical of volume-sensitive outwardly rectifying Cl(-) channel currents, including outward rectification, voltage-dependent inactivation gating at large positive potentials, inhibition by osmotic shrinkage, sensitivity to classic Cl(-) channel blockers, and dependence on cytosolic ATP. Staurosporine, but not Fas ligand or TNF-alpha, rapidly (within 30 min) increased the intracellular level of reactive oxygen species (ROS). A ROS scavenger and an NAD(P)H oxidase inhibitor blocked the current activation by staurosporine but not by Fas ligand or TNF-alpha. A ROS scavenger also inhibited apoptotic volume decrease, caspase-3 activation, and apoptotic cell death induced by staurosporine. Thus, it is concluded that an apoptosis-triggering anion conductance is carried by the volume-sensitive outwardly rectifying Cl(-) channel and that the channel activation on apoptotic stimulation with staurosporine, but not with Fas ligand or TNF-alpha, is mediated by ROS.
Figures


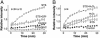


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

