Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo
- PMID: 15100256
- PMCID: PMC3752606
- DOI: 10.4049/jimmunol.172.9.5194
Inflammatory cytokines overcome age-related defects in CD4 T cell responses in vivo
Abstract
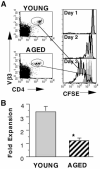
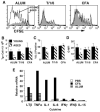
Age-related decreases in immune function are thought to contribute to the reduced efficacy of vaccinations seen in elderly populations. Our previous in vitro studies demonstrated that naive CD4 T cells from aged TCR-transgenic mice proliferate less than young cells and generate poorly functioning effectors due to decreased IL-2 production. In this current study, we show that this age-related defect in CD4 T cell response also occurs in vivo and that it is correlated with reduced NF-kappa B activation. After transfer to young hosts, CD4 T cells from aged transgenic mice proliferate less and produce reduced levels of IL-2 upon immunization with Ag and alum. Introducing a combination of the inflammatory cytokines TNF-alpha, IL-1, and IL-6, or the use of an adjuvant such as CFA that induces these cytokines, markedly enhanced responses of these aged CD4 T cells, so that they proliferated and produced IL-2 similar to young cells. This enhancement is correlated with the enhanced activation of the transcription factor NF-kappa B in aged cells. We suggest that induction of inflammatory cytokines via adjuvants may enhance the efficacy of vaccinations in elderly populations.
Figures

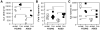

Similar articles
-
Role for IL-10 in suppression mediated by peptide-induced regulatory T cells in vivo.J Immunol. 2003 Feb 1;170(3):1240-8. doi: 10.4049/jimmunol.170.3.1240. J Immunol. 2003. PMID: 12538682
-
Role of NFATx (NFAT4/NFATc3) in expression of immunoregulatory genes in murine peripheral CD4+ T cells.J Immunol. 2003 Mar 15;170(6):3109-17. doi: 10.4049/jimmunol.170.6.3109. J Immunol. 2003. PMID: 12626567
-
Functional responses and costimulator dependence of memory CD4+ T cells.J Immunol. 2000 Jan 1;164(1):265-72. doi: 10.4049/jimmunol.164.1.265. J Immunol. 2000. PMID: 10605020
-
From naive to effector--alterations with aging.Immunol Rev. 1997 Dec;160:9-18. doi: 10.1111/j.1600-065x.1997.tb01023.x. Immunol Rev. 1997. PMID: 9476661 Review.
-
The effect of age on the cognate function of CD4+ T cells.Immunol Rev. 2005 Jun;205:220-8. doi: 10.1111/j.0105-2896.2005.00255.x. Immunol Rev. 2005. PMID: 15882356 Free PMC article. Review.
Cited by
-
Effect of polysaccharides from a Korean ginseng berry on the immunosenescence of aged mice.J Ginseng Res. 2018 Oct;42(4):447-454. doi: 10.1016/j.jgr.2017.04.014. Epub 2017 May 31. J Ginseng Res. 2018. PMID: 30337804 Free PMC article.
-
Epigenomic variability is associated with age-specific naïve CD4 T cell response to activation in infants and adolescents.Immunol Cell Biol. 2023 May;101(5):397-411. doi: 10.1111/imcb.12628. Epub 2023 Mar 1. Immunol Cell Biol. 2023. PMID: 36760028 Free PMC article.
-
Serological response to trivalent inactivated influenza vaccine in HIV-infected adults in Singapore.Hum Vaccin Immunother. 2017 Mar 4;13(3):551-560. doi: 10.1080/21645515.2016.1246636. Epub 2017 Feb 17. Hum Vaccin Immunother. 2017. PMID: 28277090 Free PMC article.
-
Immunosenescence and Challenges of Vaccination against Influenza in the Aging Population.Aging Dis. 2012 Feb;3(1):68-90. Epub 2011 Sep 30. Aging Dis. 2012. PMID: 22500272 Free PMC article.
-
Serum from exercising humans suppresses t-cell cytokine production.Cytokine. 2007 Nov;40(2):75-81. doi: 10.1016/j.cyto.2007.08.008. Epub 2007 Oct 4. Cytokine. 2007. PMID: 17919919 Free PMC article.
References
-
- Phair J, Kauffman A, Bjornson A, Adams L, Linnemann C. Failure to respond to influenza vaccine in the aged: correlation with B-cell number and function. J. Lab. Clin. Med. 1978;92:822. - PubMed
-
- Musher DM, Chapman AJ, Goree A, Jonsson S, Briles D, Baughn RE. Natural and vaccine-related immunity to Streptococcus pneumoniae. J. Infect. Dis. 1986;154:245. - PubMed
-
- Cook JM, Gualde N, Hessel L, Mounier M, Michel JP, Denis F, Ratinaud MH. Alterations in the human immune response to the hepatitis B vaccine among the elderly. Cell. Immunol. 1987;109:89. - PubMed
-
- Burns EA, Lum LG, L’Hommedieu G, Goodwin JS. Specific humoral immunity in the elderly: in vivo and in vitro response to vaccination. J. Gerontol. 1993;48:B231. - PubMed
-
- Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol. Rev. 1997;160:63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

